
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
- tutorial/specimens
- Paramecium
- Chlamydomonas
- fixing/observingflagella
- Chaos (Pelomyxa) carolinensis
- Naegleria gruberi
- the five kingdoms
Lab part 2
- experiment introduction
- microtubules
- amputating flagella
- experimental design
- data collection
- class data
Studies on Paramecium
Culture
Our laboratory usually maintains cultures of Paramecium caudatum, P. multimicronucleatum, and P. bursaria. Paramecium caudatum are the best known of the genus, however we have found P. multimicronucleatum to multiply faster (they appear to be a little bigger than P. caudatum as well). Cultures keep very well in a finger bowl covered with a watch glass to prevent evaporation. We keep the cultures in 10% Ward's basic culture solution, diluted with spring water (Ward's Biology, Rochester, NY), with pH adjusted to 7.
We provide a food source for most species by occasionally throwing in a couple of boiled wheat seeds to maintain a food chain. P. bursaria harbor photosynthetic endosymbionts, and need only be placed in a bright light. Wheat germ is a rich nutrient source for the prokaryotes that we call bacteria (Kingdom Monera). Individual monerans are single cells with no nucleus or internal organelles. The bacteria serve as a food source for small members of the phylum Mastigophora (Kingdom Protista), called Chilomonas. Chilomonas are ovate in shape, 20-40 um long.
In common with all protists, individual Chilomonas are single cells, but are distinguished from monerans by having internal organelles, including a cell nucleus. In common with all mastigophora, Chilomonas have flagella, which are long hair- or whip-like extensions of the cell that contain a small amount of cytoplasm and a core of specialized microtubules. Bacteria also have flagella, but they are not really homologous to eukaryotic flagella. Bacterial flagella are composed of the protein flagellin, not microtubules, and they are shaped like a 20 nanometer-thick, hollow, helical tube. Eukaryotic flagella propel cells by a whiplike motion derived from active processes throughout a flagellum's length. A bacterial flagellum is rotated in a corkscrew-like manner by a molecular "motor" at its base.
Chilomonas are a major food source for larger protists, including Amoeba proteus and species of Paramecium.
Aside from forgetting to maintain them, the biggest threat to our cultures are contamination with rotifers. Rotifers are animals, in fact. They are multicellular with segmented bodies and specialized cell types. They are readily distinguished from protists by their complex structure and variety of means of locomotion. They even have a primitive digestive system.
To prevent contamination of a protist culture, wheat seeds should be boiled and handled with forceps, preferably sterilized or wiped clean with alcohol. We recommend handling culture material with plastic transfer pipets, and do not recommend using the same pipet for more than one culture.
Concentrated Paramecium
It is convenient to use concentrated Paramecium in Vaseline mounts, especially in teaching labs. Students are more enthusiastic about the work if they don't have to look very hard to find specimens. A single drop of concentrated Paramecium from a 9" pasteur pipet might contain 20-30 cells. Concentrated Paramecium are also used to feed the predatory ciliate Didinium.
One way to concentrate Paramecium is to centrifuge the culture medium at a few hundred x g, preferably using conical tubes and a swinging bucket rotor. We seem to get better results, though, by transferring culture from near the wheat germ or from the bottom edges of the dish where the cells are most concentrated. Populations become very dense around and under wheat germ within a few days of adding fresh seeds.
Observation
Ciliates are capable of very fast movement, so a key to studying living ciliates is to find a way to slow them down. Protists are best observed live, in fact, species identifications frequently rely on observations of movement or characteristics that are difficult or impossible to see in fixed specimens. One devious way to slow down paramecium is to prepare a vaseline mount of concentrated Paramecium with one or more Chaos (Pelomyxa) carolinensis. Paramecium are somehow attracted to Chaos, which frequently ingests the curious. You can examine the cilia and organelles of Paramecium as the cells hover near the ameoba.
Another technique is to prepare a wet mount of the ciliates with yeast that have been stained with dye. We mix a few grams of granular baker's yeast into 100 ml warm spring water and allow the yeast to rehydrate. We then add a pinch of Congo red dye (use 0.3 mg/ml if you must have a precise formula) and heat-kill the suspension by boiling for ten minutes or so or placing the flask in a hot autoclave without running it. Heat also reduces the volume, concentrating the stained yeast. Boiling can be problematic, since violent eruptions are probable, making a mess. Even with heating to less than the boiling point, we tend to lose considerable volume due to evaporation. If the volume is reduced to 20-30 ml the density should be about right. Alternatively one can pipet from the bottom of the flask after letting the material settle.
To prepare the wet mount we place a drop of concentrated Paramecium and a drop of yeast suspension side by side on a slide so that they contact each other when we press down the vaseline coverslip. The wet mounts last longest if there is very little air under the coverslip, and the cells are easier to observe if the space between slide and coverslip is kept small. That is, use a thin layer of vaseline. The cells can be crushed, but it takes an effort to do so with a vaseline coverslip.
Within a few minutes the ciliates slow down to feed on clumps of yeast. Depending on the density of yeast particles they may move to the periphery of the mount where they can be found in clusters next to the vaseline seal. Congo red dye is a pH indicator, going from red above pH 5 to purple, then blue below pH 3. The food vacuoles change color as the pH changes during digestion of the yeast.
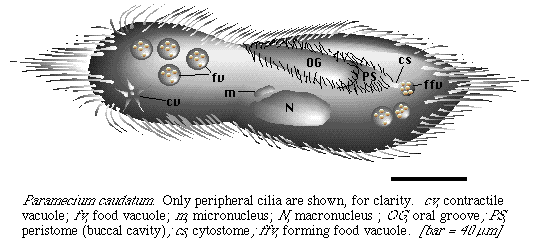
You will need to use phase contrast or dark field optics to see the cilia, which are easiest to detect at the ends of the cells and near the buccal cavity (peristome). See if you can make out how the cilia are arranged, and how they are used to propel the cell. Do the cells appear to have a sense of direction? Is there a definite dorsal vs. ventral surface? How about a front and back? Phase contrast at 400x has worked best for observing cilia, although at that magnification most of a cell will be out of focus. Phase contrast or dark field at a lower magnification also reveals cilia and organelles. Bright field is needed to distinguish colors of food vacuoles. Paramecium are so large that the cells are easily found in bright field without using high contrast.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University Dates
