
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Error Analysis and Significant Figures
Errors using inadequate data are much less than those using no data at all.
C. Babbage]
No measurement of a physical quantity can be entirely accurate. It is important to know, therefore, just how much the measured value is likely to deviate from the unknown, true, value of the quantity. The art of estimating these deviations should probably be called uncertainty analysis, but for historical reasons is referred to as error analysis. This document contains brief discussions about how errors are reported, the kinds of errors that can occur, how to estimate random errors, and how to carry error estimates into calculated results. We are not, and will not be, concerned with the “percent error” exercises common in high school, where the student is content with calculating the deviation from some allegedly authoritative number.
You might also be interested in our tutorial on using figures (Graphs).
Significant figures
Whenever you make a measurement, the number of meaningful digits that you write down implies the error in the measurement. For example if you say that the length of an object is 0.428 m, you imply an uncertainty of about 0.001 m. To record this measurement as either 0.4 or 0.42819667 would imply that you only know it to 0.1 m in the first case or to 0.00000001 m in the second. You should only report as many significant figures as are consistent with the estimated error. The quantity 0.428 m is said to have three significant figures, that is, three digits that make sense in terms of the measurement. Notice that this has nothing to do with the "number of decimal places". The same measurement in centimeters would be 42.8 cm and still be a three significant figure number. The accepted convention is that only one uncertain digit is to be reported for a measurement. In the example if the estimated error is 0.02 m you would report a result of 0.43 ± 0.02 m, not 0.428 ± 0.02 m.
Students frequently are confused about when to count a zero as a significant figure. The rule is: If the zero has a non-zero digit anywhere to its left, then the zero is significant, otherwise it is not. For example 5.00 has 3 significant figures; the number 0.0005 has only one significant figure, and 1.0005 has 5 significant figures. A number like 300 is not well defined. Rather one should write 3 x 102, one significant figure, or 3.00 x 102, 3 significant figures.Absolute and relative errors
The absolute error in a measured quantity is the uncertainty in the quantity and has the same units as the quantity itself. For example if you know a length is 0.428 m ± 0.002 m, the 0.002 m is an absolute error. The relative error (also called the fractional error) is obtained by dividing the absolute error in the quantity by the quantity itself. The relative error is usually more significant than the absolute error. For example a 1 mm error in the diameter of a skate wheel is probably more serious than a 1 mm error in a truck tire. Note that relative errors are dimensionless. When reporting relative errors it is usual to multiply the fractional error by 100 and report it as a percentage.
Systematic errors
Systematic errors arise from a flaw in the measurement scheme which is repeated each time a measurement is made. If you do the same thing wrong each time you make the measurement, your measurement will differ systematically (that is, in the same direction each time) from the correct result. Some sources of systematic error are:
- Errors in the calibration of the measuring instruments.
- Incorrect measuring technique: For example, one might make an incorrect scale reading because of parallax error.
- Bias of the experimenter. The experimenter might consistently read an instrument incorrectly, or might let knowledge of the expected value of a result influence the measurements.
It is clear that systematic errors do not average to zero if you average many measurements. If a systematic error is discovered, a correction can be made to the data for this error. If you measure a voltage with a meter that later turns out to have a 0.2 V offset, you can correct the originally determined voltages by this amount and eliminate the error. Although random errors can be handled more or less routinely, there is no prescribed way to find systematic errors. One must simply sit down and think about all of the possible sources of error in a given measurement, and then do small experiments to see if these sources are active. The goal of a good experiment is to reduce the systematic errors to a value smaller than the random errors. For example a meter stick should have been manufactured such that the millimeter markings are positioned much more accurately than one millimeter.
Random errors
Random errors arise from the fluctuations that are most easily observed by making multiple trials of a given measurement. For example, if you were to measure the period of a pendulum many times with a stop watch, you would find that your measurements were not always the same. The main source of these fluctuations would probably be the difficulty of judging exactly when the pendulum came to a given point in its motion, and in starting and stopping the stop watch at the time that you judge. Since you would not get the same value of the period each time that you try to measure it, your result is obviously uncertain. There are several common sources of such random uncertainties in the type of experiments that you are likely to perform:
- Uncontrollable fluctuations in initial conditions in the measurements. Such fluctuations are the main reason why, no matter how skilled the player, no individual can toss a basketball from the free throw line through the hoop each and every time, guaranteed. Small variations in launch conditions or air motion cause the trajectory to vary and the ball misses the hoop.
- Limitations imposed by the precision of your measuring apparatus, and the uncertainty in interpolating between the smallest divisions. The precision simply means the smallest amount that can be measured directly. A typical meter stick is subdivided into millimeters and its precision is thus one millimeter.
- Lack of precise definition of the quantity being measured. The length of a table in the laboratory is not well defined after it has suffered years of use. You would find different lengths if you measured at different points on the table. Another possibility is that the quantity being measured also depends on an uncontrolled variable. (The temperature of the object for example).
- Sometimes the quantity you measure is well defined but is subject to inherent random fluctuations. Such fluctuations may be of a quantum nature or arise from the fact that the values of the quantity being measured are determined by the statistical behavior of a large number of particles. Another example is AC noise causing the needle of a voltmeter to fluctuate.
No matter what the source of the uncertainty, to be labeled "random" an uncertainty must have the property that the fluctuations from some "true" value are equally likely to be positive or negative. This fact gives us a key for understanding what to do about random errors. You could make a large number of measurements, and average the result. If the uncertainties are really equally likely to be positive or negative, you would expect that the average of a large number of measurements would be very near to the correct value of the quantity measured, since positive and negative fluctuations would tend to cancel each other.
Estimating random errors
There are several ways to make a reasonable estimate of the random error
in a particular measurement. The best way is to make a series of measurements
of a given quantity (say, x) and calculate the mean ![]() ,
and the standard deviation
,
and the standard deviation 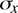 from
this data. The mean is defined as
from
this data. The mean is defined as
![]()
where xi is the result of the ith measurement and N is the number of measurements. The standard deviation is given by
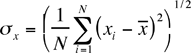
If a measurement (which is subject only to random fluctuations) is repeated
many times, approximately 68% of the measured valves will fall in the
range ![]() .
.
We become more certain that ![]() ,
is an accurate representation of the true value of the quantity x the
more we repeat the measurement. A useful quantity is therefore the standard
deviation of the mean
,
is an accurate representation of the true value of the quantity x the
more we repeat the measurement. A useful quantity is therefore the standard
deviation of the mean ![]() defined
as
defined
as ![]() . The quantity
. The quantity ![]() is
a good estimate of our uncertainty in
is
a good estimate of our uncertainty in ![]() .
Notice that the measurement precision increases in proportion to
.
Notice that the measurement precision increases in proportion to ![]() as
we increase the number of measurements. Not only have you made a more
accurate determination of the value, you also have a set of data that
will allow you to estimate the uncertainty in your measurement.
as
we increase the number of measurements. Not only have you made a more
accurate determination of the value, you also have a set of data that
will allow you to estimate the uncertainty in your measurement.
The following example will clarify these ideas. Assume you made the following five measurements of a length:
Length (mm) |
Deviation from the mean |
||
22.8 |
0.0 |
||
23.1 |
0.3 |
||
22.7 |
0.1 |
||
22.6 |
0.2 |
||
23.0 |
0.2 |
||
sum |
114.2 |
0.18 |
sum of the squared deviations |
divide by 5 |
divide by 5 and |
(N = number data points = 5) | |
mean |
22.8 |
0.19 |
standard deviation |
divide by |
|||
0.08 |
standard deviation of the mean |
Thus the result is 22.84 ± .08 mm. (Notice the use of significant figures).
In some cases, it is scarcely worthwhile to repeat a measurement several times. In such situations, you often can estimate the error by taking account of the least count or smallest division of the measuring device. For example, when using a meter stick, one can measure to perhaps a half or sometimes even a fifth of a millimeter. So the absolute error would be estimated to be 0.5 mm or 0.2 mm.
In principle, you should by one means or another estimate the uncertainty in each measurement that you make. But don't make a big production out of it. The essential idea is this: Is the measurement good to about 10% or to about 5% or 1%, or even 0.1%? When you have estimated the error, you will know how many significant figures to use in reporting your result.
Propagation of errors
Once you have some experimental measurements, you usually combine them according to some formula to arrive at a desired quantity. To find the estimated error (uncertainty) for a calculated result one must know how to combine the errors in the input quantities. The simplest procedure would be to add the errors. This would be a conservative assumption, but it overestimates the uncertainty in the result. Clearly, if the errors in the inputs are random, they will cancel each other at least some of the time. If the errors in the measured quantities are random and if they are independent (that is, if one quantity is measured as being, say, larger than it really is, another quantity is still just as likely to be smaller or larger) then error theory shows that the uncertainty in a calculated result (the propagated error) can be obtained from a few simple rules, some of which are listed in Table 1. For example if two or more numbers are to be added (Table 1, #2) then the absolute error in the result is the square root of the sum of the squares of the absolute errors of the inputs, i.e.
if ![]()
then 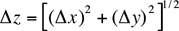
In this and the following expressions, ![]() and
and ![]() are
the absolute random errors in x and y and
are
the absolute random errors in x and y and ![]() is
the propagated uncertainty in z. The formulas do not apply to
systematic errors.
is
the propagated uncertainty in z. The formulas do not apply to
systematic errors.
The general formula, for your information, is the following;
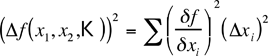
It is discussed in detail in many texts on the theory of errors and the analysis of experimental data. For now, the collection of formulae in table 1 will suffice.
Table 1: Propagated errors in z due to errors in x and y. The errors in a, b and c are assumed to be negligible in the following formulae.
Case |
Function | Propagated error |
1) |
z = ax ± b | |
2) |
z = x ± y | |
3) |
z = cxy | 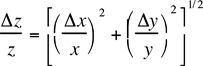 |
4) |
z = c(y/x) | 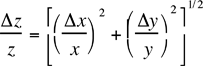 |
5) |
z = cxa | |
6) |
z = cxayb | 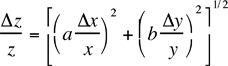 |
7) |
z = sinx | |
8) |
z = cosx | |
9) |
z = tanx |
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University Dates
