
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Detection and Measurement of Radioactivity
Radioactive Decay
Isotopes of a given element have nuclei with the same number of protons but different numbers of neutrons. Some isotopes are stable, however radioisotopes are unstable and disintegrate, with the emission of three main types of radiation.Alpha emitters release a particle composed of 2 neutrons and 2 protons. The atomic number is therefore reduced by 2, and the atomic mass by 4. Alpha particles are so heavy that even with low velocity their momentum is high. They don't travel far, but when they collide with other molecules they do a lot of damage, therefore alpha emitters are considered to be quite hazardous.
When a beta particle emitter decays, one of its extra neutrons is converted to a proton, increasing its atomic number by 1 without changing its atomic mass. The breakdown is accompanied by the emission of a negatively charged particle of low mass, called the beta particle, and an uncharged particle of low mass, called a neutrino. For example, hydrogen consists of just one proton and one electron. Deuterium (2-H), a component of "heavy water," consists of a proton, an electron, and one neutron, and is a stable isotope. Tritium (3-H) is an unstable isotope of hydrogen, consisting of a proton, an electron, and two neutrons. When an atom of tritium decays, one of the neutrons is converted to a proton, one beta particle and one neutrino are released, and a helium isotope (3-He) remains. Tritium is called a "soft" beta emitter, because its beta particles have relatively low velocities. A hard beta emitter such as 32-P (phosphorous) is more dangerous because its beta particles carry more kinetic energy (however it is easier to detect - read on).
Gamma rays consist of electromagnetic radiation resembling X-rays. An example of a gamma emitter is 131-I (iodine). Gamma radiation may accompany either alpha or beta particle emission.
A traditional unit of radioactivity is the Curie (Ci), which is defined as that quantity of any radioisotope undergoing 2.22 x 10^12 atomic disintegrations per minute (DPM). A milliCurie (mCi) of a radioisotope undergoes 2.22 x 109 DPM, and a microCurie produces 2.22 x 10^6 DPM. Since 1975, the becquerel (Bq) has replaced the Curie as the preferred international unit of radioactivity. One Bq is defined as one atomic disintegration per second, or 2.703 x 10^-11 dpm. Working amounts in a laboratory might be described in microCuries or milliCuries, kiloBecqerels or megaBecquerels.
When the product of an atomic disintegration is a stable isotope, atomic decay leaves less radioactive material behind. Therefore, as time passes, the amount of activity declines logarithmically. The half-life of a radioisotope is the time it takes for one-half of the unstable atoms to disintegrate. Each radioisotope has a characteristic rate of decay and pattern of radiation. For example, 14-C is a low energy beta emitter with a half life of 5500 years.
A radioisotope, or any compound that contains a radioisotope, is said to be radiolabeled and is called a radionuclide. The specific activity of a radionuclide is the amount of radioactivity, Bq or Ci, per mole of the compound. Clearly, as radioactive decay proceeds, the specific activity of any radionuclide declines.
Methods of detection
The method employed to detect radiation depends on the type of emitter and the intended purpose of detection. The most well known method of detecting radiation is with an ionization chamber. A high energy particle can dislodge electrons from the atoms it strikes, producing pairs of ions. Particles are allowed to pass between parallel plates, one with a positive charge and one with a negative charge. As ionization takes place the ions each move to the plate with the opposite charge, producing a current. The current is read on a meter. The Geiger-Mueller counter is based on the ionization detection principle.Photographic film can be exposed by all types of radiation, and is used to monitor exposure of personnel working with high energy emitters. A visible track in a cloud or bubble chamber can pick up radioactivity, as can a calorimeter if the energy emitted is quite high.
A problem with many methods of detection is that the energy of the emitter must be high enough to travel some distance through air. The particles emitted by many radionuclides, especially 14-C and 3-H labeled compounds, do not travel a significant distance in air, but pose a danger if internalized because of their proximity to molecules such as DNA. For example, a beta particle emitted by tritium cannot penetrate a sheet of paper, yet tritium in the body fluids can pose a significant hazard. A liquid scintillation detector can pick up radiation from "soft" beta emitters as well as from other radioisotopes. An ionizing particle is allowed to pass through a crystal or liquid phosphor, which absorbs its energy and re-emits the energy as flashes of light. Usually the emitter must be dissolved in liquid containing the luminescent compound, so that the distance traveled by the particle is very short.
Liquid scintillation counting
The amount of kinetic energy in a beta particle differs from one decay to the next. However, each radioisotope has a typical energy spectrum, that is, a predictable range of energies. Typical energy spectra for different radioisotopes can be radically different in shape and magnitude, as with the commonly used 14-C and 3-H.
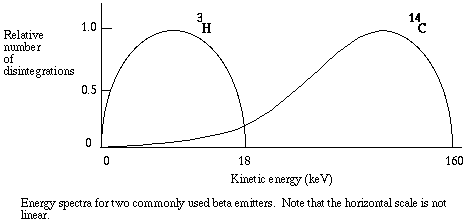
In liquid scintillation counting, the material containing radioisotopes is dissolved in an organic solvent containing an aromatic solute (the scintillant). When radioactive decay takes place, the energy of a beta particle is transferred by collision to an electron in the shell of the scintillant, exciting that electron. The electron then returns to its ground state, releasing a photon. The energy transfer can be from beta particle to solvent to scintillant, or directly to the scintillant, and usually there are multiple collisions per b particle. The number of photons emitted following each atomic disintegration is proportional to the energy of the released beta particle.
Now here is how the detection system works. The vial is lowered into a dark chamber with photoelectric detectors on each side. Each "flash" received by the detectors corresponds to one atomic disintegration. The detectors are connected, via photomultiplier tubes, to a microprocessor unit that records not only each event, but also the number of photons detected during each event (brightness of the flash). At brief intervals, such as 1/100 second, the instrument calculates the number of flashes per unit time, displaying them as counts per minute, or CPM.
Background and Quenching
No detection system perfect, of course. In liquid scintillation counting, cosmic rays, beta particles from decaying potassium in the glass vial, spontaneous discharges from the very sensitive photodetectors, and chemicals dissolved in the scintillation fluid all can contribute to spontaneous flashes of light that are recorded as counts. The CPM attributable to such sources are called background. Background counts are often so low relative to the activity being measured that they are ignored. However if the number of "real" counts is low, background counts can contribute to experimental error. It is a good practice to include a vial containing everything except added radioactivity as a control to determine the background level. Background CPM are then subtracted directly from the CPM for the experimental samples.Unfortunately, there are two more little problems. No instrument is capable of recording all of the atomic disintegrations within a scintillation vial. Because of the geometry of the vial and photoelectric detectors some events go undetected. The maximum efficiency with which a low energy emitter such as tritium can be detected is about 70%. Worse yet, the energy of some photons is absorbed by chemicals in the solvent before the photon can reach the detector. The latter phenomenon is known as quenching. With the chemical quenching that is typical of most experiments, the usual counting efficiency for tritium is 30 to 40%, and sometimes much less. The amount of quenching can vary from sample to sample, therefore it is often necessary to estimate the efficiency of counting for each individual sample.
Remember that the amount of light detected is proportional to the energy of the beta particle that was released by the disintegrating nucleus. When you prepare to count samples, you select appropriate "windows", that is, ranges of light intensity that the instrument will record as counts. The instrument records the amount of light detected following an event, and if that amount is within the energy range for a particular window, the event is recorded as one count. It is ignored if it falls outside the selected range. Each window is given a channel number, and the count for each window is given as CPM for the corresponding channel.
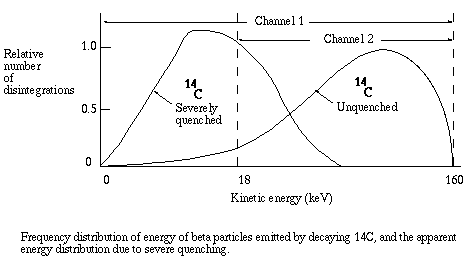
As quenching takes place, the energy recorded for each event is less than it would be for an unquenched sample, since for each event the energy of some photons is absorbed before detection is possible. The energy spectrum is shifted to the left, and the greater the quenching the greater the shift. As the shift takes place, the ratio of counts in channel 2 to counts in channel 1 becomes smaller. That ratio is known as the sample channels ratio, or SCR.
Counting efficiency is positively related to the SCR. To get a conversion factor between SCR and efficiency, equal known amounts of the isotope are added to a series of vials. Progressively greater amounts of a quenching agent, such as carbon tetrachloride, are added to each vial. The vials are counted and CPM is divided by known DPM to get the fractional efficiency of counting. For example, if a vial with 20,000 DPM of tritium yields 5,000 CPM, the fractional efficiency of counting was 5,000/20,000 = 0.25, that is, 25% of the atomic disintegrations were detected. Fractional efficiency is plotted versus SCR to yield a quench curve. The instrument prints out CPM and SCR for each sample, therefore to get actual DPM in a sample the investigator must (1) subtract background CPM from the CPM for the sample, (2) determine fractional efficiency from the SCR for the sample, and (3) divide net CPM by the fractional efficiency.
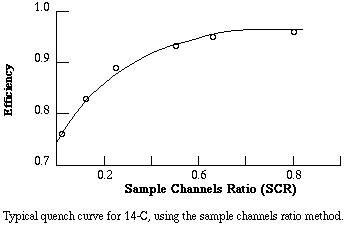
Example. You are counting samples containing 14-C. Sample #1 gives you channel 1 CPM of 1323. The background counts were 23 CPM and the SCR was 0.5. From the quench curve, an SCR of 0.5 corresponds to an efficiency of 0.93. Then the estimated amount of radioactivity in the sample is (1323 - 23)/0.93 = 1398 DPM.
There are other problems associated with the measurement of radioactivity that are not so easily solved. However, for single label experiments the quench correction is all you need. Review the special safety cautions for radioisotope work, and you are ready to go.
The scintillation counter printout
Modern scintillation counters have a conveyor system that automatically feeds samples in order into the counting chamber. As each sample is counted the relevant information is printed. A typical printout includes preliminary information followed by specific information on a sample by sample basis. Every manufacturer uses a different system of terms and abbreviations, so either you will need access to the instrument manual or the printout from your instrument will have to be translated by an experienced individual.Preliminary information may include the date, time, user i.d., and program selected. Common counting parameters are usually listed. The parameters may include: number of times each sample is counted; time period of counting each sample; number of times the entire set of samples is counted; type of quench correction; windows selected; special features such as criteria for cutting short a count if counts are very high, or automatic background subtract. The preliminary information must indicate that the counting parameters were appropriate for your type of samples.
The information supplied for each sample typically includes: sample i.d., which may be position number in a rack, position in order of counting, or both; channel numbers (windows) and corresponding counts per minute; time of counting; elapsed time since the run was started (this is important for radioisotopes with very short half lives); sample channels ratio or other measure of quenching.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 7 Sep 95
Updated 10 Aug 12s
