
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
- tutorial/specimens
- Paramecium
- Chlamydomonas
- fixing/observingflagella
- Chaos (Pelomyxa) carolinensis
- Naegleria gruberi
- the five kingdoms
Lab part 2
- experiment introduction
- microtubules
- amputating flagella
- experimental design
- data collection
- class data
An Amoebo-flagellate: Naegleria gruberi
Naegleria species are members of Class Zoomastigophorea, which includes the amebomastigotes. The latter name is derived from the common name for many members of Phylum Sarcodina (the amoebae and relatives), and Phylum Mastigophora, the flagellates.The puzzling life cycle of Amebomastigotes led to the term 'amebo-flagellate,' which describes the two known active stages. The transformation of amoeboid forms into flagellates was described by F. Schardinger (1899). He was working with an organism he called Amoeba lobosa, which he isolated from feces. He found that they formed cysts - dormant forms - under certain conditions. He also described a problem with 'flagellate nuisances' that kept appearing in the water droplets that appeared due to condensation in his agar dishes. His attempts to find some developmental stage of the flagellates were fruitless, resulting always in a plate full of uniform cysts. By accident, he inoculated a hanging drop with amoebae, and two hours later discovered that they had been almost entirely replaced with fast swimming flagellates. Schardinger had performed the first recorded Naegleria transformation experiment.
Amebomastigotes are very common soil protists that have been isolated from soil and fresh water, and occasionally from marine water and sediments throughout the world. Many members of Class Zoomastigophorea are pathogenic. Some strains of Naegleria are deadly, but fortunately they are uncommon. Naegleria fowleri, for example, can contaminate fresh water and infect human hosts while the latter are swimming or bathing. While in the flagellate state they enter through the nose and parasitize the brain in the amoeba form. The result is amoebic meningoencephalitis, which is nearly always fatal. Fortunately, Naegleria gruberi is not known to be pathogenic to humans, and the commonly used laboratory strain (strain NEG) is harmless.
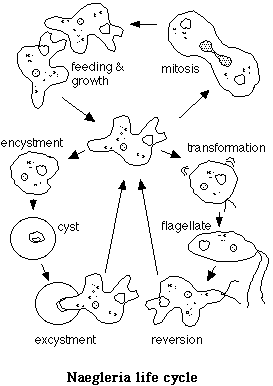
Upon transfer to liquid media from a solid substrate, Naegleria amoebae usually undergo a cellular differentiation process in which they change from a crawling amoeba to a streamlined swimming flagellate. The organelles move about in an amoeba, but become immobilized in the flagellate. All of the organelles of the flagellar apparatus must be formed and extensive construction of cytoskeletal structures must take place during the transition. Only the amoebic form is capable of feeding, and reproduction is by mitosis of the amoeba. The flagellate reverses the process after about two hours, reverting to the amoebic form. The exact conditions that trigger the transformation are unknown.
Experiments with Naegleria
Here is one important note. Do not attempt to culture Naegleria from a natural source. They are easily recognized in water samples, and chances are they are nonpathogenic, but why take chances? For our studies we use N. gruberi, strain NEG, from the American Type Culture Collection. It takes about a week to obtain active amoebae from the freeze dried cysts.
Our cultures feed on the bacterium Xanthomonas Maltophilia, although other bacteria would probably do as well. We propagate cultures as described in Fulton, Chandler: Amebo-flagellates as Research Partners: The Laboratory Biology of Naegleria and Tetramitus. Meth. Cell Physiol. 4: 341, 1970. Agar media consist of (gms/liter), Bacto-peptone 2.0, dexrose, 2.0, anhydrous potassium phosphate dibasic 1.5, anhydrous potassium phosphate monobasic 1.0, Bacto agar 20. Medium should be stirred to distribute the agar prior to pouring ~40 ml per 100 mm dish (autoclave the medium with stir bar).
For quick propagation of amoeba one can prepare a suspension by "hosing" down the surface of an agar culture with sterile distilled water, inoculating agar plates with 1-2 ml suspension. The suspension can then be distributed using a glass spreder. It is critical that plates not be allowed to dry. Sealing with parafilm usually does the trick. For short term maintenance or to check viability of a rejuvenated culture one should produce a bacterial lawn was produced by spreading bacteria suspension on an agar plate and allowing it to develop for a day or two at room temp. A loopful of amoebae from plate or suspension can then be used to inoculate one edge of the plate by making a line of 1-2 cm in the lawn. Amoebae form a recognizable plaque on successful edge plates, which are then either sealed with Parafilm and stored refrigerated or used to prepare suspensions.
Agar slant tubes can be used for long term maitenance. Viable cysts last for months at room temperature in slant tubes with caps closed. Tubes hold moisture better than plates, which is critical since none of the stages of Naegleria tolerate dessication.
Naegleria transformation
When Naegleria amoebae are faced with specific environmental and/or internal cues, they differentiate into flagellates. Mass plates, on which most of the bacteria are cleared by feeding amoebae, are the best source of suspensions. Naegleria cysts are harvested by washing down a mass plate with distilled water or 2 mM Tris buffer, pH 7.5 and suspension transferred to a 15 ml capped conical disposable centrifuge tube. Amoeba can be separated from remaining bacteria by pelleting for 2-3 mn at 400 x g. If desired, cell density can be determined with the aid of a hemocytometer.
The Fulton article recommended the use of a hanging drop for following the transformation of amoeba to flagellate. The transformation does take place consistently within a vaseline mount, although not all amoebae transform. Preparations should be checked frequently at low and high powers, using phase contrast and/or dark field to spot swimming flagellates. The latter swim toward the top of the chamber, so that if the observer is focused at high power on the amoebae the flagellates may not be evident. Examples of all stages of the life cycle should be evident in control cultures starting from cysts.
Inhibition of transformation
Since the development of paired flagella is a part of the Naegleria transformation from amoeba to flagellate, it seemed appropriate to look at the effects of inhibitors of flagellar regeneration in transforming amoebae. Since transforming amoebae are differentiating cells, it would be expected that a protein synthesis inhibitor would have a profound effect on development, beyond simply inhibiting flagellar growth. To the extent that microtubules are involved in the organization of intracelluar structures we might predict a similar response to the presence of colchicine.
The initial experiment simply involved harvesting amoebae or cysts, then suspending them in 10 micrograms/ml emetine or cycloheximide, 3 mg/ml colchicine, or medium only. Control and experimental cultures were mounted in vaseline chambers and their progress followed for a couple of hours. Systematic methods of recording results were followed. For example, a set of random high power microscope fields were examined at half-hour intervals and scored for numbers of cysts, amoebae, and transformants.
The results were surprising the first time we conducted this study. The responses to the inhibitors were more complex than in Chlamydomonas, as expected, but there were also some completely unexpected results. For example, depending on the time at which suspensions were treated, the transformation process was accelerated by colchicine. This surprising effect was seen when the cultures consisted of active amoebae at the time of resuspension. This study points out that to study specific cellular mechanisms one should start out with the simplest possible model and work up. Obviously it is not a good idea to study the regulation of microtubule assembly using a model organism that differentiates during an experiment.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University Dates
