Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
Lab part 2
Lab part 3
Selected methods
SDS-PAGE "Hall of Shame"
It doesn't matter if you fall
down as long as you pick something up from the
floor when you get up.
Efraim Racker
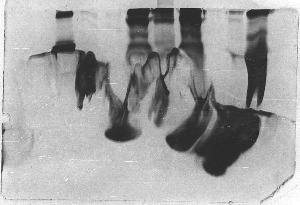
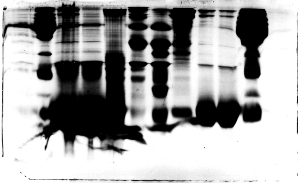
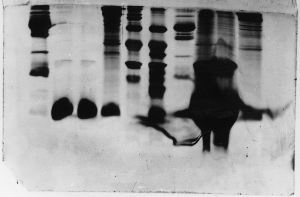
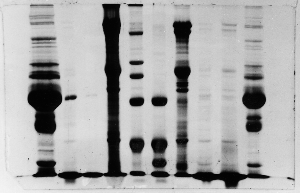
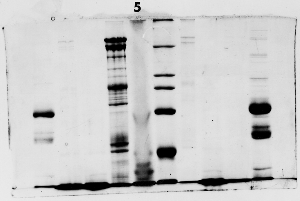
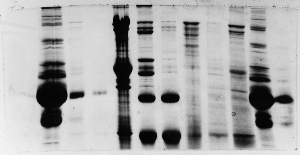
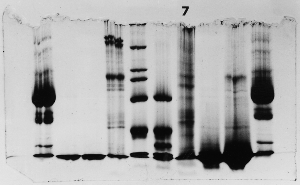
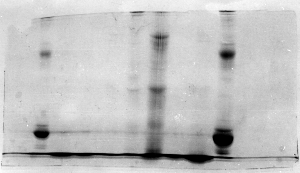
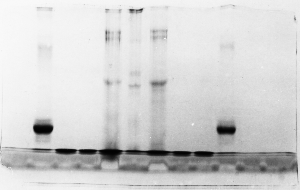
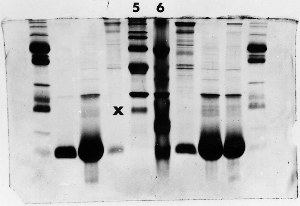
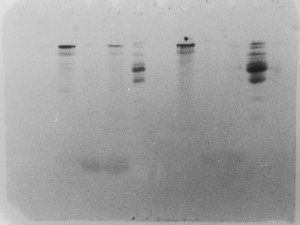
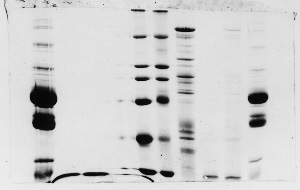
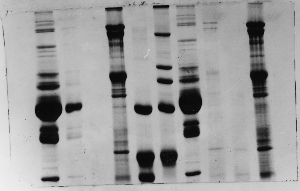
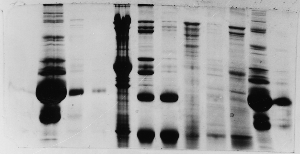
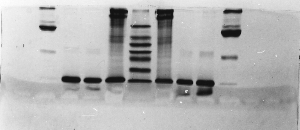
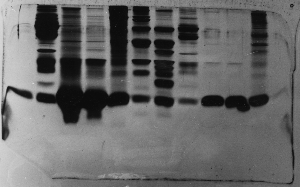
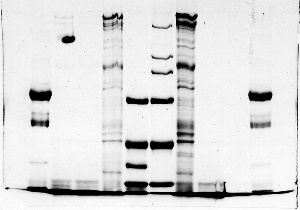
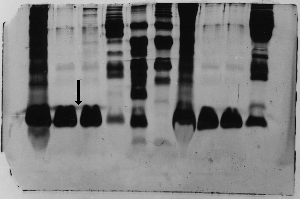
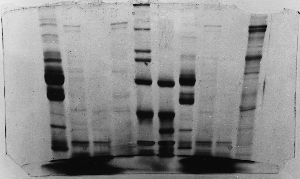
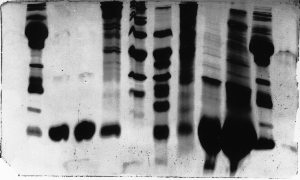
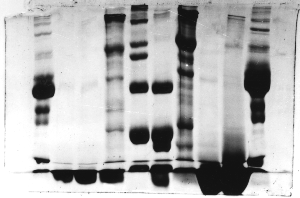
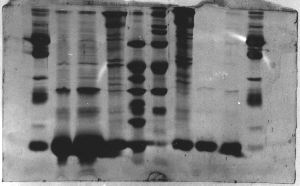
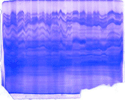
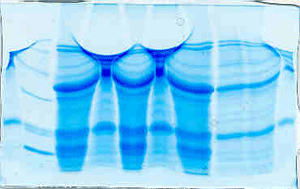
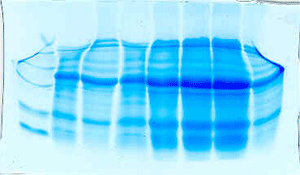
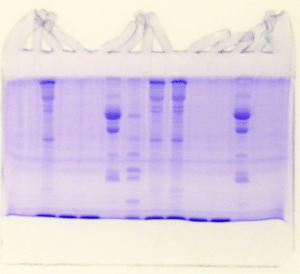
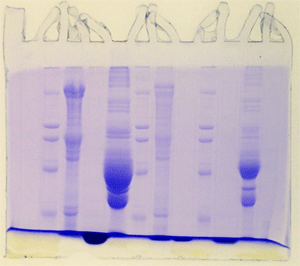
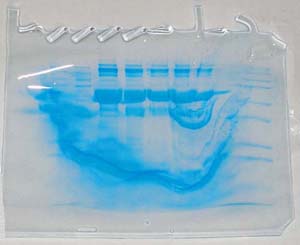
The "Hall of Shame" presents examples of some of the worst gels students (and instructor) have run in past labs, with an example or two from a research lab. They represent many of the ways one can mess up a gel (but not all of them - we're still finding new ways!). See what features of your own gel(s) were unsatisfactory - or at least less than perfect - and use the illustrations to figure out what you might do to improve your technique.
Examples of past gels that didn't quite work out
To critique your own work identify your symptoms and use the gallery to select appropriate example(s). Each example is linked to a full sized image with suggessted explanations for the symptoms. From this "hall of shame" you should be able to determine what can be done to correct the problem(s). If you wish you may browse by descriptions of symptoms, listed below the gallery.
Gallery
Symptoms
Smears
[Bad pour] [Gross overloading] [Overloaded, nonuniform gel] [Failure to denature sample]
Streaks
[Particles in sample] [Overloading] [Top of gel uneven]
Bands too light
[Sloppy loading] [Bad stain] [Overestimated protein concentration] [Forgot to stain]
No dye front
[Partial dye front] [No dye front with distortion] [No dye front with frown]
Bands only at top of gel
[Stopped early, diffuse dye front] [Stopped early, tight dye front]
Miscellaneous
[Crooked bands] [Horizontal lines] [No penetration] [Broken gel] [Field effects]
Multiple symptoms
[No dye front, crooked, overloaded] [Crooked, field effects, overloaded] [Torn gel, uneven loading]
Bizarre results
[Wrong electrode buffer] [Overheating] [Delay in running gels] [Moldy buffer] [Opened gel box during the run!]
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 9 Oct 96
Updated 28 Nov 06