
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
Lab part 2
Lab part 3
Selected methods
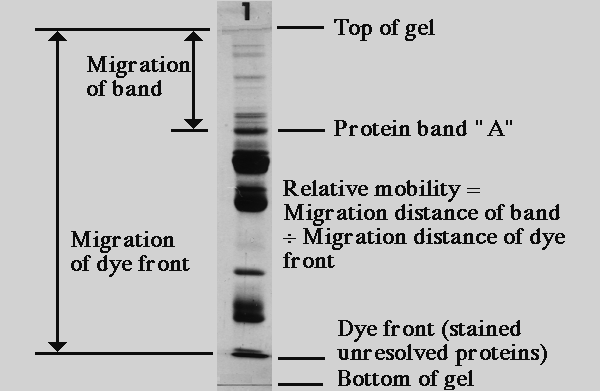
Measuring Relative Mobility of Protein Bands
The term relative mobility refers to the movement of a type of polypeptide through a gel relative to other protein bands in the gel. Relative mobility is the distance migrated by a band divided by the distance migrated by the dye front. Absolute mobility would be the distance traveled in a particular time. Relative mobility is useful because it can be used to compare the migration of a protein from gel to gel, regardless of the physical length of the gel or duration of electrophoresis. A common abbreviation for relative mobility is Rf (the f is a subscript), for "retention factor."
The use of relative mobillity for estimating protein mass stems from the days when each sample was run on a separate gel, in tubes rather than on a slab. Each sample and set of standards had its own dye front. The migration distance varied from one sample or set of standards to the next, but as long as the identical batch of acrylamide gel mix was used for each tube, the relative mobility of a given polypeptide was the same for each separate tube. When multiple samples are run on a slab gel with standards, it is not necessary to calculate relative mobility. If one does not need to compare mobility from one gel to another, it suffices to measure migration distance only.
Relative mobility of a polypeptide band is related to its molecular mass. By running a set of protein standards of known molecular mass, a standard curve of mass versus relative mobility can be produced, from which estimates of apparent mass can be made for unknown proteins.
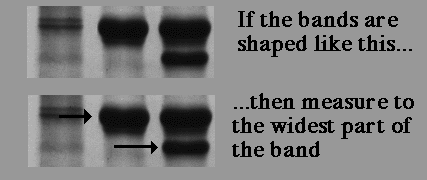
If the band is 'fat,' meaning there is too much protein, either find the same protein in a smaller quantity in another lane, re-do the gel with less protein, or estimate the relative mobility without doing another gel (my first choice, since the apparent MW is only an estimate anyway). Students often ask where to measure... middle of the band? Top of the band?

How gel concentration affects relative mobility
Each polyacrylamide gel effectively separates proteins over a specific and limited molecular weight range. There are two reasons for the limitations. First, the acrylamide concentration determines the cut-off at the low end. All polypeptides below a minimum molecular weight run at the same pace as the tracking dye. They do not separate from each other, and are therefore not resolved at all. Second, the relationship between mass and relative mobility is logarithmic. Resolution of individual bands tends to diminish toward the top of a gel, so that with the more dense gels multiple bands may appear to merge into a single band. Usually the top 10% or more of a gel is unusable.So what happens if you want to characterize all of the proteins in a sample? ...run more than one gel, of course! A gel with low density will resolve the larger polypeptides while cutting off the lighter ones, and one of higher density will reveal the smaller polypeptides, while compressing and possibly distorting the larger ones.
Here are some examples of the effect of acrylamide concentration on relative mobility. Molecular weight standards are identified by number. A typical erythrocyte membrane protein sample is also presented, with band 3 protein labeled as a reference.
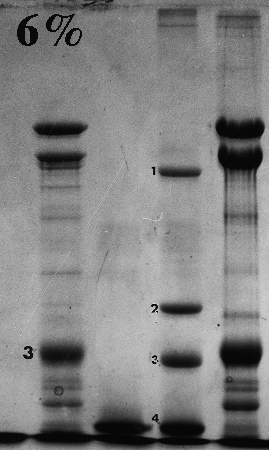
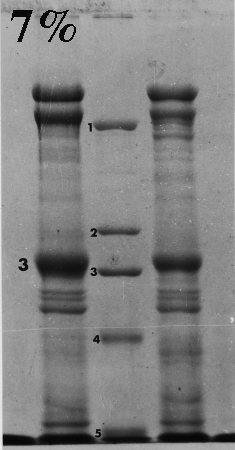
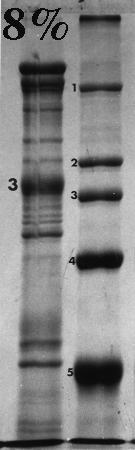
Standard 1 = myosin (205,000); std. 2 = beta-galactosidase (116,000); std. 3 = phosphorylase B (92,000); std. 4 = bovine serum albumin (66,000); std. 5 = egg albumin (45,000); std. 6 = carbonic anhydrase (29,000).
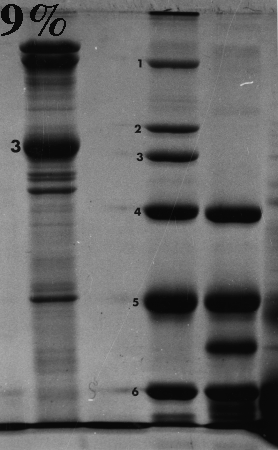
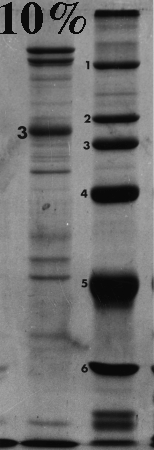
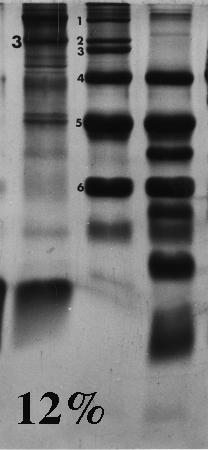
On the gels from 6 to 10% there is a distinct dark doublet at the top of the membrane lane. Notice in the 12% gel the doublet is jammed together and appears as one band. One could estimate MW of band 3 from the first five gels although the best estimate comes from the 6%, which produced the greatest separation between the standards on either side of band 3. The 12% gel did not resolve bands well at all above the fourth standard (serum albumin, 66,000). Analysis should be confined to the part of the gel below 66,000 or even lower if the same sample was run on a lower density gel.
Frequently, students analyze the upper part of a high density acrylamide gel that overlaps part of a lower density gel. That practice suggests that the student missed the point of the anaysis and/or did not understand the limitations of the method. Interpret only that part of the denser gel that doesn't overlap the other one. To put it another way, use high density gels to study proteins (or parts of proteins) of relatively low molecular weight, and lower density gels to resolve proteins of higher molecular weight.
Don't forget that different polypeptides can have similar or even identical molecular masses. One band on a gel can therefore consist of one or more polypeptides. This is most likely to happen toward the top of a gel, and especially in higher density gels.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 19 Aug 96
Updated 5 Jan 07
