Failed Gels - Crooked, Broken, Smeared, Weird
Crooked bands
Examples of this problem are scattered throughout
the 'hall of shame.' The surface of the resolving
gel was uneven, so that when the samples were stacked,
the bands started out with distorted shapes.
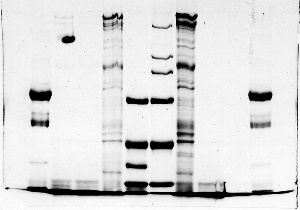
Crooked bands also result from an uneven electric
field, but usually that type of distortion is symmetrical.
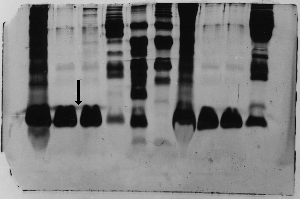
Bad wells
If samples run over into adjacent wells, you will
see what looks like a continuous protein band running
across the wells (arrow). In fact, that's exactly
what it is. This is usually what happens when the
wells are too short, or perhaps sample is accidentally
spilled outside the wells.
In addition, we may have used old sample buffer.
An advantage of using Cleland's reagent (dithithreitol,
or DTT) to reduce disulfide bonds is that it doesn't
stink as badly as 2-mercaptoethanol. However, the
latter compound is much more stable. Disulfide
bonds link individual polypeptides together and
maintain some proteins in a folded state under
otherwise denaturing conditions. This causes them
to run unpredictably.
Dense Gel
...or should it be dense investigators?
The gel mix was clearly incorrect. The acrylamide
concentration was so high that only the smallest
proteins entered the gel to any extent. The
bands were distorted due to overloading and
the fact that beyond a reasonable concentration
the most concentrated gels tend not to polymerize
evenly.

Broken Gel
This can be really frustrating.
You did a very nice job, then the gel broke
during handling. Keep in mind that the gel
will still be usable as long as you save
the pieces.

Field Effects
To some extent this is normal,
although for some reason the field effect was
exaggerated here. The spacers interrupt the
electric field at the edges of the gel, so
that the 'pull' (or 'push' if you prefer) is
biased to one side the closer the sample is
to the edge of the gel. The result is the 'frowning'effect
you see here.
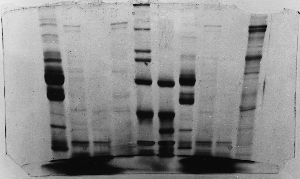
The individual bands sometimes
smile, but if the gels have any expression
at all, they always frown. I guess you would
too if we zapped you with electricity and drowned
you in blue dye.
|


![]()











