
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Introduction/
training
Mitochondria theory
Mitochondria in vitro
- preparation
- fate of substrates
- state IV
- state III
- metabolic poisons
- mitotraces
- rationale
- experiments
Additional topics
Polarographic System for Measurement of Dissolved Oxygen
Studies in modern biological science frequently require instrumentation, for measurements such as changes in a quantity with time. A basic recording setup includes a measurement device, a processing or coupling device, and a recorder. In a polarographic system the measurement device (transducer) is a Clark oxygen electrode. The processing/coupling device is an oxygen monitor. The recording device is a flatbed strip chart recorder, or perhaps a computer-assisted data acquisition system.The solution or suspension to be assayed is placed in a sealed chamber that is exposed to the surface of a Clark oxygen electrode. This dissolved oxygen (D.O.) chamber contains one or more access ports for adding/removing materials. The medium is stirred to ensure homogeneity and to ensure that oxygen can freely diffuse into the electrode. The presence of oxygen causes the electrode to deliver a current to the oxygen monitor, which amplifies the current and converts it to a voltage output that is directly proportional to the concentration of oxygen in the chamber. The recorder moves a paper chart at constant speed, so that when the recorder pen moves in response to voltage changes, oxygen content is recorded as a function of time.
For mitochondria studies our medium of choice consists of 70 mM sucrose, 220 mM mannitol, 2 mM HEPES buffer, 5 mM magnesium chloride, 5 mM potassium phosphate, 1 mM EDTA, and 0.1% fatty acid free bovine serum albumin, pH 7.4. In our results we refer to the rate at which total chamber oxygen declines as the oxygen consumption rate.
Recommended components
For polarographic systems we use custom made glass Gilson type DO chambers that hold a nominal volume of 2 ml. Gilson type chambers are designed with water jackets, however we haven't found it necessary to regulate temperature in our studies. We use the smallest availble "flea sized" stirring bars (maximum 5/16" l x 1/16" dia) to maintain laminar flow in the chambers. For dissolved oxygen detection we use Yellow Springs Instruments (YSI) #5331 Clark electrodes withYSI Model 5300 two channel biological oxygen monitors. We record data on Kipp & Zonen BD single channel chart recorders.
Principle of the Clark oxygen electrode
A Clark oxygen electrode is composed of two half cells separated by a salt bridge. A platinum electrode is separated from a solid silver electrode by insulating material. A concentrated potassium chloride solution is held in place over the surfaces of the electrodes by a teflon membrane which is attached by an O-ring that surrounds the electrodes. The oxygen monitor holds a constant voltage difference across the two electrodes so that the platinum electrode is negatively charged with respect to the silver electrode.
Platinum is a strong catalyst for the covalent dissociation or reassociation of water. In the Clark electrode, electrons "boil" off of the platinum electrode, combining with dissolved molecular oxygen and hydrogen ions to produce water. The rate at which electrons boil off is proportional to the concentration of oxygen that is available to "grab" them. The movement of electrons is an electrical current, of course, which is then converted to a voltage by the oxygen monitor circuitry as mentioned above.
Biologists are often happy to leave the details to the physical scientists. However, successful biologists know the principles behind their instrumentation so that they are prepared to troubleshoot instrumentation or even modify a system to fit their needs. Here is how the reactions balance. Current flows from the silver electrode to the platinum electrode as electrons boil off into solution from the latter. Removal of electrons from solid silver produces silver ions. The silver ions combine with chloride ions in solution to precipitae silver chloride on the surface of the silver electrode. This leaves potassium ions behind, however since hydrogen ions are taken out of solution by the consumption of oxygen, the charge remains balanced.
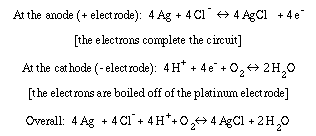
Some Facts about Dissolved Oxygen
On earth, at standard temperature and pressure the proportion of oxygen in air, by volume, is 20.9% (at least for now). In an aqueous solution that is allowed to equilibrate with room air, oxygen again constitutes 20.9% of the total dissolved gas. In both air and in solution, the balance is mostly nitrogen, with some carbon dioxide present as well.
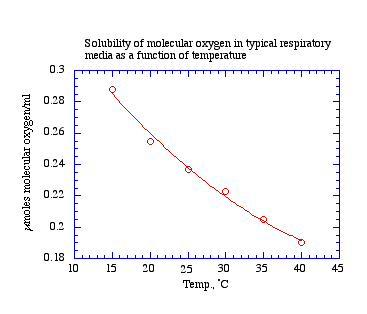
The concentration of dissolved gas in solution varies with temperature, and may be markedly affected by solutes and other solvents present in solution. For example, a warm solution holds less oxygen than a cold solution. The presence of ethanol significantly increases the capacity of an aqueous solution to hold oxygen. In your studies, temperature variation in the room may affect equilibration of your media with room air and the stability of the records. Some reagents that you add to the chamber will contain ethanol.
A buffered solution of mitochondria respiration medium (e.g., 70 mM sucrose, 220 mM mannitol, 2 mM HEPES buffer, 5 mM magnesium chloride, 5 mM potassium phosphate, 1 mM EDTA, and 0.1% fatty acid free bovine serum albumin, pH 7.4), equilibrated with room air, typically holds 0.237 micromoles molecular oxygen per ml at 25 degrees C. Knowing the volume of medium in the chamber, one can calculate the total oxygen. From the records, the investigator can calculate how much oxygen in micromoles is used up per unit time.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 12 Dec 96
Updated 30 Jan 08
