
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Introduction/
training
Mitochondria theory
Mitochondria in vitro
- preparation
- fate of substrates
- state IV
- state III
- metabolic poisons
- mitotraces
- rationale
- experiments
Additional topics
The Electron Transport System of Mitochondria
Embedded in the inner membrane are proteins and complexes of molecules that are involved in the process called electron transport. The electron transport system (ETS), as it is called, accepts energy from carriers in the matrix and stores it to a form that can be used to phosphorylate ADP. Two energy carriers are known to donate energy to the ETS, namely nicotine adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). Reduced NAD carries energy to complex I (NADH-Coenzyme Q Reductase) of the electron transport chain. FAD is a bound part of the succinate dehydrogenase complex (complex II).
It is reduced when the substrate succinate binds the complex.What happens when NADH binds to complex I? It binds to a prosthetic group called flavin mononucleotide (FMN), and is immediately re-oxidized to NAD. NAD is"recycled," acting as an energy shuttle. What happens to the hydrogen atom that comes off the NADH? FMN receives the hydrogen from the NADH and two electrons. It also picks up a proton from the matrix. In this reduced form, it passes the electrons to iron-sulfur clusters that are part of the complex, and forces two protons into the intermembrane space.
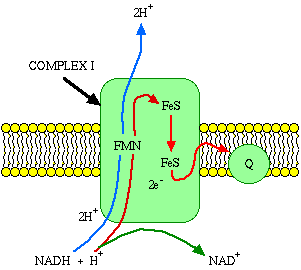
The obligatory forcing of protons into the intermembrane space is a key concept. Electrons cannot pass through complex I without accomplishing proton translocation. If you prevent the proton translocation, you prevent electron transport. If you prevent electron transport, you prevent proton translocation. The events must happen together or not at all.
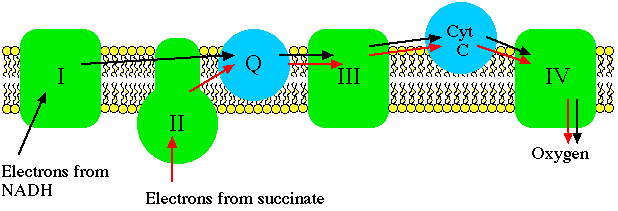
Electron transport carriers are specific, in that each carrier accepts electrons (and associated free energy) from a specific type of preceeding carrier. Electrons pass from complex I to a carrier (Coenzyme Q) embedded by itself in the membrane. From Coenzyme Q electrons are passed to a complex III which is associated with another proton translocation event. Note that the path of electrons is from Complex I to Coenzyme Q to Complex III. Complex II, the succinate dehydrogenase complex, is a separate starting point, and is not a part of the NADH pathway.
From Complex III the pathway is to cytochrome c then to a Complex IV (cytochrome oxidase complex). More protons are translocated by Complex IV, and it is at this site that oxygen binds, along with protons, and using the electron pair and remaining free energy, oxygen is reduced to water. Since molecular oxygen is diatomic, it actually takes two electron pairs and two cytochrome oxidase complexes to complete the reaction sequence for the reduction of oxygen. This last step in electron transport serves the critical function of removing electrons from the system so that electron transport can operate continuously.
The reduction of oxygen is not an end in itself. Oxygen serves as an electron acceptor, clearing the way for carriers in the sequence to be reoxidized so that electron transport can continue. In your mitochondria, in the absence of oxygen, or in the presence of a poison such as cyanide, there is no outlet for electrons. All carriers remain reduced and Krebs products become out of balance because some Krebs reactions require NAD or FAD and some do not. However, you don't really care about that because you are already dead. The purpose of electron transport is to conserve energy in the form of a chemiosmotic gradient. The gradient, in turn, can be exploited for the phosphorylation of ADP as well as for other purposes. With the cessation of aerobic metabolism cell damage is immediate and irreversible.
From succinate, the sequence is Complex II to Coenzyme Q to Complex III to cytochrome c to Complex IV. Thus there is a common electron transport pathway beyond the entry point, either Complex I or Complex II. Protons are not translocated at Complex II. There isn't sufficient free energy available from the succinate dehydrogenase reaction to reduce NAD or to pump protons at more than two sites.
Is the ETS a sequence?
Before the development of the fluid mosaic model of membranes, the ETS was pictured as a chain, in which each complex was fixed in position relative to the next. Now it is accepted that while the complexes form 'islands' in the fluid membrane, they move independently of one another, and exchange electrons when they are in mutual proximity. Textbooks necessarily show the ETS as a physical sequence of complexes and carriers. This has the unintentional effect of implying that they are all locked in place. The fluid nature of membranes allows electron exchange to take place in a test tube containing membrane fragments.
The location of ETS complexes on the inner membrane has two major consequences. By floating in two-dimensional space, the likelihood of carriers making an exchange is much higher than if they were in solution in the three dimensional space of the matrix. They are exposed to the matrix side of the membrane, of course, for access to succinate and NADH, but have limited mobility. Second, the location of the ETS on the inner membrane enables them to establish a chemiosmotic gradient.
Electron pathways and inhibition
Electron transport inhibitors act by binding one or more electron carriers, preventing electron transport directly. Changes in the rate of dissipation of the chemiosmotic gradient have no effect on the rate of electron transport with such inhibition. In fact, if electron transport is blocked the chemiosmotic gradient cannot be maintained. No matter what substrate is used to fuel electron transport, only two entry points into the electron transport system are known to be used by mitochondria. A consequence of having separate pathways for entry of electrons is that an ETS inhibitor can affect one part of a pathway without interfering with another part. Respiration can still occur depending on choice of substrate.
An inhibitor may competely block electron transport by irreversibly binding to a binding site. For example, cyanide binds cytochrome oxidase so as to prevent the binding of oxygen. Electron transport is reduced to zero. Breathe all you want - you can't use any of the oxygen you take in. Rotenone, on the other hand, binds competitively, so that a trickle of electron flow is permitted. However, the rate of electron transport is too slow for maintenance of a gradient.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 12 Dec 96
Updated 31 May 05
