
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Introduction/
training
Mitochondria theory
Mitochondria in vitro
- preparation
- fate of substrates
- state IV
- state III
- metabolic poisons
- mitotraces
- rationale
- experiments
Additional topics
Chemiosmotic Gradient: Generation and Maintenance
Energetics of proton translocation at Compex I
The energy needed to push protons out of the matrix and into the intermembrane space comes from the oxidation of either reduced NAD (NADH) or reduced FAD (FADH2). In the case of NADH, passage of an electron pair to Coenzme Q provides a "pulling" force. That is, NADH is much less electronegative than Coenzyme Q, while the iron-sulfur protein carriers in between are intermediate in electronegativity. The amount of available free energy is 69.5 kJ/mole of NADH (kiloJoules per mole). The efficiency of electron transport can be represented by the standard reduction potential difference, namely the voltage generated by a redox reaction under standard biochemical conditions.
The standard reduction potential of NADH is -0.315V, while that of coenzyme Q is 0.045V (difference of 0.345 V). Therefore there is a strong 'pull' by Coenzyme Q on electrons through the components of Complex I.
Just for the sake of understanding the principles, let Complex I (NADH dehydrogenase complex), embedded in an intact inner membrane, be the only component of an experimental electron transport system. We'll simply take the electrons from Coenzyme Q when they reach it, so the system can keep going. The removal of protons from the matrix and deposition of protons in the intermembrane space creates a concentration difference of protons across the inner membrane. This is called the chemiosmotic gradient. As the gradient builds up, more and more energy is required to push protons across. When the amount of energy required to push protons reaches 69.5 kJ/mole, electron transport has to stop. In fact, the second law of thermodynamics requires that electron transport stop before the gradient builds up to that point.
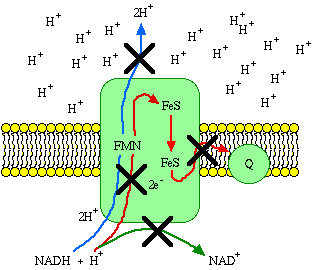
If there was no way of draining energy from the system, electron transport could not continue despite the presence of adequate substrate. However, a mitochondrion is always in a steady state of respiration, in which the energy lost by processes that dissipate the gradient is constantly replaced by electron transport.
Respiratory Control
The limitation placed on electron transport by the chemisosmotic gradient is termed respiratory control. Mitochondria are said to exercise respiratory control as long as they can restrict electron transport by means of the gradient. If the gradient is destroyed by damaging the membranes, respiratory control is abolished and electron transport can run freely.
Cause and effect relationship
Think of physical causes and effects when you attempt to describe respiratory control. What drives electron transport? It can't be driven by a "need" to maintain the gradient, because that implies a sense of purpose. The electron transport system is just a structure, complex as it is. Electron transport is driven by the increasing affinities of successive carriers for electrons, and by the availability of substrates to provide electrons and free energy. It is restricted by the chemiosmotic gradient - electron transport can only go as fast as energy is lost from the gradient. Anything that increases turnover of energy from the gradient increases the rate of electron transport proportionally.
Here is an analogy that might help with the concept. Suppose that you are trying to blow up a balloon. Blowing with all your might you succeed in filling the balloon so that it is two feet in diameter. You cannot force any more air into it so all you are doing now is holding pressure. Now some joker comes along and pokes a couple of holes in your balloon, so that air leaks out at a controlled rate. You have maintained constant pressure, so now you find yourself moving air into the balloon as it leaks out. The balloon diameter, proportional to internal pressure, remains constant. If you plug one of the holes you move less air. Open up more holes and you move more air. The electron transport system applies constant "pressure," holding the gradient at a constant level. The rate of electron tranport (analagous to air flow in the balloon example) varies as energy is drained from the system at different rates. Adding ADP in vitro, for example, opens up avenues for protons to be forced into the matrix, draining energy from the gradient. Its addition is the equivalent of poking additional holes in your balloon. Electron transport spontaneously increases.
Respiratory Control in Exercise
Sometimes in wading through all of the details, one loses sight of the big picture. Just why is all of this information about electron transport and oxidative phosphorylation so important, anyhow? Suppose you are vegging in front of the TV - in the prone position with a bag of cholesterol chips. You are very relaxed and are hardly aware that you are breathing at all. Suddenly the fire alarm sounds, and a bunch of naked people are running down the hall. This definititely provokes activity on your part, and you run down the hall also. You start breathing heavily (due to the exercise, not the sight of the naked people).Your demand for energy is rather low when you are relaxed. However running is heavy exercise. Your muscle activity causes an immediate demand for ATP which is met in part by muscle glycogen. When that runs low you need to replace your reserves with aerobic metabolism, that is, your mitochondria need to make more ATP. Electron transport is stimulated when the ratio of ATP to ADP goes down. The rate of binding of ADP to the ATP synthetase automatically increases as more ADP is transported into the matrix. In turn, electron transport is allowed to speed up - consuming more oxygen in the process. Sensors in your cardiovascular system (primarily the carotid bodies of the carotid arteries) detect an increase in carbon dioxide (indicating an increased need for oxygen). The sensors send a signal to the brain via the nervous system that you need to speed up breathing.
So... you breathe primarily to get oxygen into the blood and to the tissues, in order to deliver oxygen to the mitochondria for cellular respiration - to make ATP. In the process, carbon dioxide is returned to the lungs and exhaled. The processes are essential to your well being, in fact, to your being at all. When you think of the effects of some of the poisons that are discussed on these pages, think of the consequences to an intact organism such as yourself.
The big picture, including regulatory pathways such as the very simplified description presented above, are in the realm of integrative disciplines such as physiology. Such disciplines are increasingly ignored in both research and academic curricula in favor of a purely molecular approach to science. Please consider the importance of knowing the consequences of molecular and cellular processes to the whole organism, and the fact that the regulatory pathways controlling those processes are still incompletely understood.
Uncoupling and the basal metabolic rate
Endotherms like ourselves (formerly called homeotherms) maintain a constant body temperature. The setpoint for that temperature is determined by a region the hypothalamus (part of the brain, for you newcomers). But just how is the temperature regulated? That is, how does the body maintain a balance between heat production and heat loss?The answer to that one is very complex. There are many mechanisms for heat dissipation and retention (sweating, changes in distribution of the circulation, shivering, piloerection - no, it's not a dirty word, etc.). Heat production results from the loss of some of the free energy of every chemical reaction as heat - that is, every chemical reaction results in the loss of some of the total free energy in the universe. That's one of the laws of thermodynamics - I forgot which. A major source of heat production is electron transport in mitochondria.
How do mitochondria produce heat? Much of the energy released by electron transport does no useful work at all (that law of thermodynamics is at work again). That is, each exchange of electrons results in a loss of some energy as heat. Furthermore, dissipation of the chemiosmotic gradient without doing work results in the transformation of a great deal more energy to heat. The mechanism of respiratory control can be exploited to increase heat production by dissipating the gradient at a faster rate, or to slow heat production by making the ETS more efficient. One example of that type of regulation is the hormone thyroxine (thyroid hormone).
Thyroid hormone has many functions, mostly associated with promotion of anabolic activity - synthesis of compounds and cellular growth. However thyroxine is also a mildly effective uncoupling agent. Uncoupling agents dissipate the chemical gradient, usually by creating a mechanism by which protons can escape the intermembrane space (or otherwise 'short out' the gradient), allowing an increase in electron transport. In colder regions of the world a noticible change in thyroid hormone levels takes place circannually, that is, levels are higher in winter and lower in summer.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 12 Dec 96
Updated 31 May 05
