
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Introduction/
training
Mitochondria theory
Mitochondria in vitro
- preparation
- fate of substrates
- state IV
- state III
- metabolic poisons
- mitotraces
- rationale
- experiments
Additional topics
Substrate Oxidation: Krebs Reactions
The citric acid cycle is also called the Krebs cycle, after Hans Krebs, who first proposed its cyclic nature. The Krebs' cycle reactions take place in the matrix of the mitochondria. Some of the final steps of intermediate metabolism take place there as well. For example, in the matrix as well as the cytoplasm, glutamate (the amino acid glutamic acid) loses its amino group and is oxidized to alpha-ketoglutarate.Most texts and lecture courses start with glycolysis, then proceed to describe Krebs' cycle as the "next step" in metabolism of sugars. Unfortunately, such a presentation may leave students with the impression that metabolites follow a linear progression through glycolysis to acetyl-coenzyme A to citric acid, through the Krebs intermediates to oxaloacetate, which is then coupled to the two carbons of another acteyl-coenzyme A to regenerate citric acid. Even in biochemistry texts, chapters on Krebs' cycle may start with a picture showing only pyruvate as the starting point.
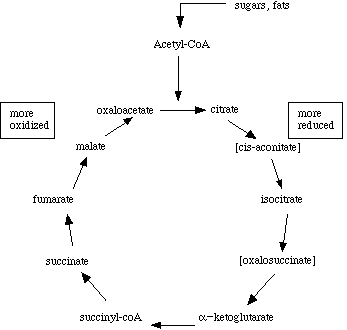
This figure is what sticks in the minds of most introductory level students, and it isn't complete at all. First, it isn't just glycolysis that leads to the generation of acetyl coenzyme A. Carbohydrates, fats, and proteins are all nutrients, and in fact fatty acid metabolism results in the generation of acetyl coenzyme A as well.
Second, the 'starting point' for Krebs' cycle need not be acetyl-coenzyme A at all. In fact, it really isn't appropriate to refer to a cycle as having a starting point (e.g., where does a circle start?). Amino acids and odd-chain fatty acids can be metabolized into Krebs intermediates, and enter at several points.
Finally, intermediates can be 'siphoned off' for use in biosynthetic pathways. They must be replaced in order to maintain energy balance, however the fate of a Krebs intermediate is not necessarily to cycle through the enzymes until it is completely oxidized to carbon dioxide and water.
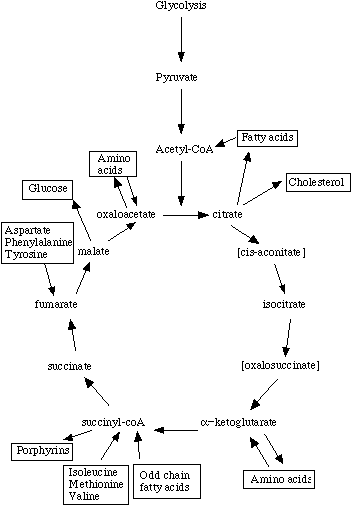
Since cycle intermediates can be incorporated into both anabolic and catabolic pathways, the cycle is really amphibolic, not just catabolic.
Glutamate to alpha-ketoglutarate
The enzyme complex known as glutamate dehydrogenase binds the glutamate molecule, a molecule of oxidized nicotine adenine dinucleotide (NAD), and a water molecule. Off comes the amino group, and glutamate is partially oxidized to alpha-ketoglutarate, which you should recognize as a Krebs intermediate. In vivo, the alpha-ketoglutarate would be further oxidized to succinyl-coenzyme A by alpha-ketoglutarate dehydrogenase, then the succinyl-coenzyme A would be oxidized to succinate, etc. However, remember the amphibolic nature of Krebs cycle - substrates may be utilized for biosynthesis rather than undergoing further oxidations. In addition, limitations on Krebs cycle in isolated mitochondria must be considered.
The oxidation of organic compounds releases free energy. This energy would be wasted as heat, except the enzyme also catalyzes the reduction of the NAD. The enzyme is designed so that the reaction cannot take place unless all of the reactants are present. Free energy from the partial oxidation of glutamate is used to reduce the NAD. The result is a molecule of NADH, which retains in its structure some of the free energy lost by the glutamate.
![]()
Glutamate dehydrogenase catalyzes a reaction in which the energy from an exothermic reaction is used to power an endothermic reaction. Therefore it performs what we call a coupled reaction. The second law of thermodynamics, paraphrased, states that some of the useful energy in any system is converted to useless energy during any process. That is, any spontaneous process causes the entropy of the universe to increase. In coupled reactions, only some of the free energy is conserved in the form of a reduced product. The remainder is released as heat.
NADH is an energy carrier. After release from glutamate dehydrogenase it can be used to power other reactions. It can also bind a specific enzyme on the mitochondria inner membrane, transferring some of its free energy to the electron transport system. In mitochondria, this is the principle role of NADH.
Succinate to fumarate
An important substrate in our studies of mitochondria function is succinic acid (succinate). When succinate is brought into or generated in the mitochondria matrix in sufficient quantity, succinate molecules bind the enzyme complex called succinate dehydrogenase. The succinate dehydrogenase complex is also known as complex II of the electron transport system, thus the oxidation of succinate to fumarate is the only Krebs reaction that takes place on the inner membrane itself, as opposed to the other reactions that are catalyzed by soluble enzymes. The energy carrier flavin adenine dinucleotide (FAD) is also a part of the succinate dehydrogenase complex.
Because the enzyme and FAD are both part of the same complex, the only step needed to initiate succinate oxidation is the binding of succinate to the enzyme. Even in severely compomised mitochondria succinate supported respiration can usually be accomplished, as long as fragments of the inner membrane remain.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 24 Apr 96
Updated 31 May 05
