
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
- tutorial/specimens
- Paramecium
- Chlamydomonas
- fixing/observingflagella
- Chaos (Pelomyxa) carolinensis
- Naegleria gruberi
- the five kingdoms
Lab part 2
- experiment introduction
- microtubules
- amputating flagella
- experimental design
- data collection
- class data
Tutorial and Specimens to Examine
Why study invertebrates in the first place? Why not focus our studies on human tissues and human diseases? It its purest form, science seeks to understand nature regardless of the potential utility of such knowledge in solving immediate or foreseeable problems. However, for you pragmatists out there you have another very good reason for studying biological models that have no apparent immediate relevance to human concerns.
An important axiom in cell biology is that different organisms solve similar problems in similar ways. As complex as you may believe yourself to be, you share the most basic processes of life with the simplest of living things. The less complex the organism, the easier it is to perform controlled experiments. You are related by evolution to all other organisms, and more often than not, there are similarities among gene sequences that are involved in a particular process that can lead you to an explanation of that very same process as it takes place in the cells of more complex organisms, including humans.
A simple cellular system can be more fully characterized than cells obtained from humans, in a reasonable time. For example, the Chlamydomonas genome is about one hundred times smaller than the human genome. It will still be a long time before all of the mechanisms of regulation of gene expression in Chlamydomonas are known, but consider how much more time it will take to fully understand the significance of all of the DNA sequences in the human genome, and how much more difficult it would be without clues from simpler organisms.
You can obtain large numbers of invertebrates, including single-celled organisms, for study. You can easily control the conditions under which they are grown and maintained. Many tissues from multicellular invertebrates do not require supplemental oxygen or complex media, unlike vertebrate tissues. Their central nervous systems are not as well-developed as those of vertebrates, therefore invertebrates are probably not self-aware. There are no ethical restrictions on their use, with the exception of concerns about pathogenicity or potential environmental hazards stemming from improper handling of potentially dangerous cultures.
All of the activities described here can be completed in one four hour session. With one or two exceptions, all of the studies involve living preparations. After all, why stop the action? In many ways we can learn much more by observing a living organism than we can by killing and preserving it.
Tutorial – using a research microscope
The tutorial uses a prepared slide of Paramecium with stained yeast. We will go over the features and care of a microscope and describe the light path. We will introduce the optical features of our microscopes and how to set them up. You will learn how to find a specimen, select appropriate optics, adjust illumination, focus, and raise magnification. You will learn how to adjust a binocular eyepiece tube and oculars to accommodate individual users, and how to adjust illumination, aperture, and phase contrast settings to optimize viewing of different specimens.
Recommended specimens
As you work, record your observations with notes, sketches, and measurements. Note how you prepared each slide, how you located and observed each specimen, and what combinations of condenser setting and objective lens worked best for each purpose. You are not required to observe all of the specimens. Required preparations are marked with an asterisk.
Time management
Plan ahead, estimating how much time you have to prepare and observe each of the specimens. Have a contingency plan in case you cannot observe them all, and save some time (five minutes or so) for finishing up the notebook. Part of the performance grade is based upon how efficiently you work, including whether or not you finish by the scheduled end of the lab period.
*Paramecium with stained yeast, supplied by the instructor
Record the name of the species, which in our laboratory can be P. caudatum, P. multimicronucleatum, or P. bursaria. Observe motility and feeding behavior. Try to identify organelles in the ciliates, estimate the diameter of a typical yeast cell, and use the ocular micrometer scale to estimate length and width of a typical Paramecium. We recommend usng 40x dark field to find the cells initially, 100x bright or dark field to observe feeding behavior and color changes in food vacuoles, and 400x phase contrast for observing cilia and yeast.
You may wonder what else you are seeing on these slides, in addition to Paramecium and the yeast cells. Paramecium are large cells as protists go, much larger than individual yeast. The web page on Paramecium (next) should give you some clues as to what is on the slide, including the small objects that are moving rapidly through the preparation.
How to Prepare a Wet Mount (Vaseline mount)
For most of the remaining specimens you will need to prepare your own slides using a technique known as a wet mount or Vaseline mount.
Living specimens do not survive long in the heat from an intense microscope illuminator bulb, usually because the specimen dries up. This problem is easily solved by preparing a vaseline chamber. Simply take a single cover slip and hold it between thumb and forefinger by the edges. Pick up some vaseline on the other forefinger and rub it over your thumb to make a film. Scrape your thumb carefully on each edge of the coverslip to make a continuous vaseline ledge.
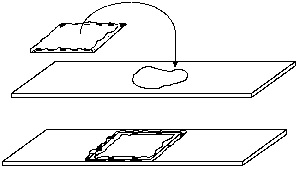
Place a drop or two of suspension on a clean slide, and turn the coverslip over on top of the drop. Press down the edges to seal the chamber against evaporation. When preparing a vaseline mount, keep in mind that the image becomes degraded with thicker mounts, especially at high powers in dark field or phase contrast. Unless the specimen is large and fragile enough to be damaged by pressing down too hard on the coverslip, keep the chamber depth very shallow.
*Living Chlamydomonas
It is critical that you learn to prepare living or fixed/stained for viewing, learn to quickly find cells and get them undr high magnification, and adjust contrast to observe and measure flagella.
Prepare a wet mount of motile Chlamydomonas reinhardi. Use your newly acquired skills to find cells and record the procedure that works best for you. Try to locate a cell that is stuck to a surface. Is the cell stuck on the slide or up on the coverslip? How can you tell?
You cannot see flagella in bright field mode, even at high magnification. Even with phase contrast, finding unstained flagella can be a challenge. Examine cells that aren't moving or that appear to be stuck to a surface, and work with the fine focus and phase controls until you can spot the hair-like organelles. Use the fine control to focus all the way through a specimen, including the full length of a flagellum. Discuss the procedure with a teaching assistant and confirm that you are indeed seeing flagella.
*Fixed and stained Chlamydomonas
Staining with iodine gives the flagella more contrast. In dark field flagella appear white against a dark background, while in phase contrast they are dark against a gray background. Staining also fixes the cells, that is, it kills them while preserving their structure.
Fix and stain a sample using 1-2 drops Lugol's iodine to 3-4 drops cell suspension. Practice finding the stained, immotile cells at low magnification, targeting specific cells for observation at high magnification, and optimizing contrast for observation and measurement of flagella. You must be prepared to recall these particular skills, since you will use them for data collection later. In your notebook, record the procedures that work best for you. Report (and record) the length of a typical flagellum.
Paramecium bursaria
Paramecium are nonphotosynthetic ciliates, yet P. bursaria are green. Where might the green color come from? Do you ever find the green structures outside the larger cell? Make up a wet mount of P. bursaria. We might have to add an agent such as "Protoslo" to get them to stop moving. After observing the living cells for awhile, try crushing them by pressing down on the coverslip. What are you seeing? Try here for an idea.
Ingestion of Paramecium by Chaos
Chaos (Pelomyxa) carolinensis are so large that they are easily crushed by a coverslip, and collecting individuals from a culture takes practice. We will provide wet mounts of Chaos in a proportion of 5-10 Paramecium to one Chaos.
The food chain in freshwater communities starts with bacteria, which are eaten by a host of organisms including small protists. Paramecium, among other protists and metazoans, feed on the protists, and lots of organisms feed on Paramecium. We might think of them as the "cattle" of freshwater laboratory cultures. They are good for maintaining larger predatory organisms, including Chaos. Chaos are huge, as much as a millimeter in diameter when they spread out on a surface. You can readily find and observe them at 40x final magnification. You might make most of your observations using 100x.
Most amoebae shy away from light. Your Chaos will likely remain stationary until it is placed in the light path in bright field. As the amoeba moves it will shoot out pseudopodia that are well attached to a glass surface (the surface could be the slide or the underside of the cover slip). Pseudopodia are very thin, so one can see a lot of detail. Try using different optics to observe motility by pseudopodium formation and cytoplasmic streaming, including phase contrast.
Try to estimate the volume of a typical Chaos carolinensis. What assumption or assumptions must be made? Can you measure a cell in three dimensions to come close to an accurate estimate? Hint: What if you know the volume of liquid that was placed on the coverslip? How would you define "accurate" in this context? Try determining the minimum and maximum possible volumes. If you had time, what might you do to narrow the range of possibilities (improve the accuracy of your estimate)? Sometimes accuracy is not possible and/or not necessary. Learn to work within limits. Consider that astronomers and cosmologists sometimes consider it to be a great accomplishment if they obtain an estimate that is accurate within an order of magnitude.
Observations on living bacteria
Prepare a Vaseline mount from a broth culture or hay infusion of living bacteria, using a very thin Vaseline layer.
Use dark field at low power to find the suspension, focusing first on a bubble or cover slip. the bacteria will be visible even at the lowest power, as bright spots against a dark background. Work up to 400 power. When you go from 100x to 400x, go to phase contrast mode, then swing the 40x phase contrast lens in place and focus. You may need to adjust the phase centering screws to get good contrast. Living non-motile bacteria exhibit Brownian motion, that is, they vibrate due to molecular motion. Motile bacteria will rapidly cross the field of view or lumber along in various directions depending on their size.
Should you expect to identify organelles in bacteria? Why or why not? Are there non-bacterial species present? How can you distinguish them from bacteria? You will need to recall information from your general biology background. Obtain help if you are having trouble answering these questions.
What criteria might you use to distinguish different species of bacteria within this mixed culture? What are typical volumes of two or more species? You will have to model a single bacterium as a straight, curved, or spiral cylinder, or as a sphere, whatever choice is appropriate.
Examination of stained bacteria
When bacteria are heat-fixed and stained they tend to clump together. At high dry power individual cells often can't be detected because the space between cell walls is within the limits of resolution of the microscope due to diffraction. To properly view stained bacteria it is necessary to use oil immersion microscopy.
Obtain a slide of gram stained bacteria. Each slide is etched on the lower surface, with the heat-killed and stained bacteria on the opposite (upper) surface. Mount the slide with smear up and focus at low power on the etching. Move the slide so that the lens is over the smear itself. Now move the objective away from the slide until the upper surface comes into focus.
A bacterial smear at low power looks like a patch of dirt. Focus on the mess, first at 40x then at 100x and finally 400x, all in bright field mode. Note that you can see some detail at 400x, but the shapes and colors of the bacteria are somewhat distorted.
Move the 400x lens out of the way, place a drop of immersion oil directly on the smear where the objective was, and swing the oil immersion lens in place. Move the fine focus up and down slightly to ensure that the lens is in contact with the oil. Now watch the end of the objective and bring it as close to the slide surface as you can without touching it. Note in which direction you must focus to move the objective away from the slide, look in the eyepieces, and slowly rotate the fine focus control until the image is focused.
Most bacterial species are rod-shaped or round (cocci), although some are curved, spiral-shaped, or irregularly shaped. The gram stain leaves some cell types pink (Gram negative) and others dark blue (Gram positive) depending on cell wall characteristics. Gram stain results are a major criterion for identification of species. Note how much more clear the image is at 1000x with oil than it was at 400x without oil.
Mixed freshwater cultures (collected specimens)
An effort will be made to provide "wild" freshwater cultures with a great diversity of species, but our success in providing interesting materials varies with time of the year and with the weather. Even when a culture is not obviously teeming with organisms, there is plenty to see if you know how to look.
Estimate the dimensions and the volumes of the largest and smallest organisms you can find. What is the order of magnitude of the difference in the long dimension (an order of magnitude is a factor of ten). or example, if the longest species is a millimeter long and the shortest is 1 micrometer (0.001 mm) long, the difference is three orders of magnitude. What is the order of magnitude of the difference in volume? See if you can estimate the order of magnitude of the difference in magnitude between the volume of the smallest organism you observe, and your own volume. You might report the quantity as the number of organisms that could fit inside you if you were hollow. Such measurements are extremely imprecise, so you may find it necessary to round both the raw measurements and the estimates of order of magnitude to a single significant digit.
For several different organisms, use your present knowledge of general biology to assign them to appropriate kingdoms. Record a description of the organism, the kingdom, and your criteria for the assignment to that kingdom
Examination of the amoebo-flagellate Naegleria gruberi
Naegleria is a soil protist that feeds in an amoeboid form for most of its life. Under some conditions the amoeba will transform into a flagellated stage. The transformation can be observed by suspending the amoebae in water then preparing a vaseline mount of the suspension. For Naegleria, the Vaseline layer should be very thin.
Locate cysts using dark field, 40x and/or 100x. You may have to use the Vaseline film or an air bubble as an intitial target. The cysts resemble perfect circles or spheres and may be clustered together like grapes. They are quite small - how small? Estimate the diameter of a cyst at 400x phase contrast or bright field. In phase constrast you should see detail on the surface of the cysts. If you see very little detail, then you may need some help in adjusting the phase contrast optics.
A little bit of scanning should turn up the amoebae, which are about the same diameter as the cysts but more irregularly shaped and moving. Can you identify organelles? What sorts of organelles should you be able to identify, that is, what are typical features of members of Kingdom Protista?
A wet mount of Naegleria will keep for hours. Observe the same slide about an hour and a half after preparation. If your mount is well sealed, it will not have dried up. Any changes? Look for rapid movement at 100x (dark field).
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University Dates
