
Home
Studies
& Data Analysis
Methods
Microscope studies
Flagella experiment
Laboratory math
Blood fractionation
Gel electrophoresis
Protein gel analysis
Mitochondria
Concepts/ theory
Keeping a lab notebook
Writing research papers
Dimensions & units
Using figures (graphs)
Examples of graphs
Experimental error
Representing error
Applying statistics
Principles of microscopy
Solutions & dilutions
Protein assays
Spectrophotometry
Fractionation & centrifugation
Radioisotopes and detection
Guide to the study
Lab part 1
- tutorial/specimens
- Paramecium
- Chlamydomonas
- fixing/observingflagella
- Chaos (Pelomyxa) carolinensis
- Naegleria gruberi
- the five kingdoms
Lab part 2
- experiment introduction
- microtubules
- amputating flagella
- experimental design
- data collection
- class data
Chlamydomonas as a Model Organism
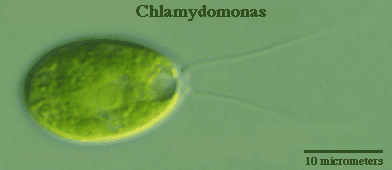
Chlamydomonas, a genus of unicellular photosynthetic flagellates, is an important model for studies of such fundamental processes as photosynthesis, motility, responses to stimuli such as light, and cell-cell recognition.C. reinhardi, the most commonly studied species of Chlamydomonas, has a relatively simple genome, which has been sequenced. Exposure to sunlight in an appropriate medium produces uniform cultures containing large numbers of motile cells. Many different strains, including nonmotile strains, have been developed for specific research purposes. Since all organisms are related by evolution, the knowledge acquired from studies of Chlamydomonas allow researchers to learn more about regulation of gene expression in more complex plants and animals.
For example, if we discover that a particular gene is involved in the regulation of microtubule assembly in a protist model we can sequence it, look for homologous sequences within the genome of a multcellular organism, and work our way up to the human genome. Usually, finding the gene responsible for a particular mechanism in human tissue without studying simpler model organisms is nearly impossible.
Chlamydomonas has served as a model for studies of the regulation of microtubule assembly. Flagella of Chlamydomonas are typical of eukaryotic cilia and flagella, in that they are composed of microtubules arranged in the well-documented "9 + 2" structure. Since different organisms solve similar problems in similar ways, studies on how microtubule assembly is regulated in Chlamydomonas may reveal mechanisms that are shared by most other organisms.
Microscopic examination and measurement of flagella
Motile Chlamydomonas can be induced to shed their flagella or flagella can be removed mechanically. Provided the cells themselves and the basal bodies on which flagella are constructed are not damaged, they re-grow flagella following such amputation. By using agents with very specific known actions to interfere with cellular processes, we can learn how cells initiate flagella growth, control the rate of growth, and establish a terminal length. Such information can then be extended to microtubule systems in more complex organisms.
A means of measuring flagellar growth is necessary. Since fairly uniform cultures can be prepared, one way of assessing growth is to take samples at specific time intervals, to preserve the cells in the exact condition they were at the time sampled, and to measure the length of their flagella using a light microscope. To stop the cells from continuing to grow flagella during the measurement (scoring) process, samples of cells should be killed and "fixed." Effective fixatives preserve structures at the microscopic level by cross-linking proteins, allowing one to make observations at a fairly leisurely pace. Unfortunately, flagella become brittle and begin breaking off a few minutes after fixing cells, thus observations on flagella must be made rather promptly.
The pigmentation and motion of living Chlamydomonas allow them to be spotted in a bright field microscope. At low power in bright field one simply focuses on the moving green objects, then moves up in magnification. They move so fast, though, that with higher magnifications it is necessary to find a cell that is stuck to a surface. Both living and fixed Chlamydomonas are much easier to find and observe if contrast is enhanced using phase contrast or dark field microscopy.
Nonmotile cells settle to the bottom of a culture tube, so the tube should be agitated before sampling fixed cells. One small drop of culture should be sufficient for preparation of a wet mount, with or without vaseline. A recommended procedure is to set up the microscope for low magnification (e.g., 100x) in phase contrast or dark field mode, whichever is available. Focus on the edge of an air bubble or visible piece of debris that is definitely between slide and coverslip. It is essential at this point to be aware of what a 10 micrometer diameter object looks like at 100x (quite small). Cells will appear as out of focus circles, ghostly in appearance. As with any specimen, the image becomes smaller and color more intense as it comes into focus.
With one or more cells centered in a field at 100x, the magnification should be increased to high dry mode (e.g., 400x) in either dark field or phase contrast. Cells can be distinguished by their size, shape, color, and presence of organelles. Flagella, if present, can then be measured using a calibrated ocular micrometer scale.
Visitors: to ensure that your message is not mistaken for SPAM, please include the acronym "Bios211" in the subject line of e-mail communications
Created by David R. Caprette (caprette@rice.edu), Rice University 28 Jun 95
Updated 12 May 05
