Bands Too Light
Light bands – example 1
It looks like this problem was caused by badly formed
wells and/or careless loading of sample onto the
gel. The background is dark and uneven, suggesting
that protein was in the running buffer itself. Most
likely the apparatus was jarred and much of the samples
was slopped out of the wells. The spilled sample
would not resolve into bands, since it continuously
entered the gel from the upper buffer compartment.
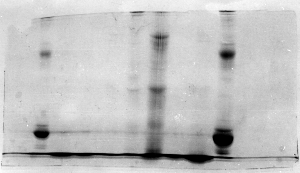
Note the presence of a horizontal line, continuous
along several lanes (arrow). That would suggest
that the wells were too shallow, and some material
spilled into adjacent wells.
This symptom also shows up in perfectly good
gels when the electrodes are hooked up backwards
for a few minutes. Protein migrates out of the
wells instead of into the stacking gel. Catching
the problem within a few minutes salvages some
of the information, but the pH effect that causes
efficient stacking is compromised, sample is lost,
and protein contaminates the running buffer.
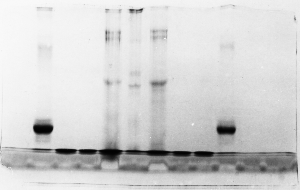
Light bands – example 2
Judging from the lack of other symptoms, it appears
that the gel simply didn't stain well. Coomassie
blue dye staining solution can become contaminated
with SDS if it is recycled. The dye becomes less
effective and proteins don't show up as well as
with fresh dye. As long as the proteins were precipitated
in the gel by the acidified alcohol in the stain
solution, the problem could be corrected simply
by re-staining with fresh dye.
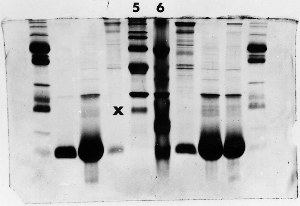
Light bands – example 3
The 'X' marks the lane next to lane 5 where the amount
of protein was overestimated in the original sample,
the sample was prepared to the wrong concentration
for electrophoresis, or too little was loaded - perhaps
sample wasn't properly drawn up into the syringe.
The absence of any major bands, compared to the other
samples, suggests that the well was simply underloaded.
Light bands – example 4
This is one of the more disappointing symptoms you
might encounter. Let's say you were extremely meticulous,
did everything perfectly, and ran your gel so that
the dye front was perfectly even and right at the
bottom. You rinsed the gel in deionized water, then
left it on the shaker. Being late in the day, you
forgot to replace the water with the stain. It was
stained the next day, but here's the result:
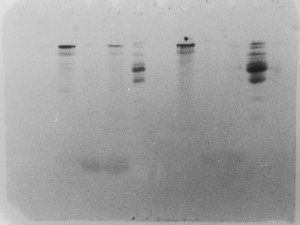
Notice that the smaller proteins diffused rather
quickly out of the gel, while the larger ones at
the top diffused much more slowly, and took the
stain. The acidified alcohol in the stain solution
and in the destain solutions is essential, as it
precipates proteins, preventing them from diffusing
out of the acrylamide matrix.
|


![]()










