Enzymatic
synthesis of an indole diterpene by an oxidosqualene cyclase:
Mechanistic, biosynthetic, and phylogenetic implications.
Xiong, Q., Zhu, X., Wilson, W.K., Ganesan,
A., and Matsuda, S.P.T. J. Am. Chem., 125 (2003): 9002-9003.
Abstract;
PDF
Mutagenesis
approaches to deduce structure-function relationships in terpene
synthases.
M. J. R. Segura, B. E. Jackson, and S. P. T. Matsuda. Nat.
Prod. Rep., 20 (2003): 304-317.
Abstract
Subcellular
localization of oxidosqualene cyclases from Arabidopsis thaliana,
Trypanosoma cruzi, and Pneumocystis carinii expressed
in yeast.
P. Milla, F. Viola, S. Oliaro-Bosso, F.
Rocco, L. Cattel, B. M. Joubert, R. J. LeClair, Matsuda, S. P.
T., and G. Balliano. Lipids, 37 (2002): 1171-1176.
Abstract
Directed
evolution experiments reveal mutations at cycloartenol synthase
residue His477 that dramatically alter catalysis.
M. J. R. Segura, S. Lodeiro, M. M. Meyer, A. J. Patel, and S.
P. T. Matsuda. Org. Lett., 4 (2002): 4459-4462.
Abstract;
PDF
Directed
evolution to generate cycloartenol synthase mutants that produce
lanosterol.
M. M. Meyer, R. Xu, and S. P. T. Matsuda. Org. Lett., 4
(2002):1395-1398.
Abstract;
PDF
Functional
cloning of an Arabidopsis thaliana a cDNA encoding cycloeucalenol
cycloisomerase.
M. A. Lovato, E. A. Hart, M. J. R. Segura, J.-L. Giner, and S.
P. T. Matsuda. J. Biol. Chem., 275 (2000): 13394-13397.
Abstract;
PDF
A
tyrosine to threonine mutation converts cycloartenol synthase
to an oxidosqualene cyclase that forms lanosterol as its major
product.
J. B. R. Herrera, W. K. Wilson, and S. P. T. Matsuda. J.
Am. Chem. Soc., 122 (2000): 6765-6766.
PDF
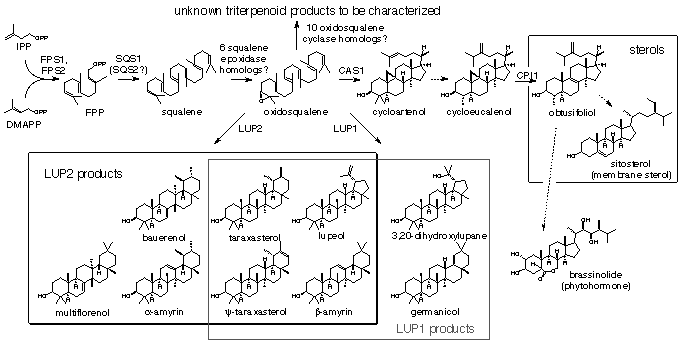
Arabidopsis
thaliana LUP1 converts oxidosqualene to multiple triterpene
alcohols and a triterpene diol.
M. J. R. Segura, M. M. Meyer, and S. P. T. Matsuda. Org.
Lett., 2 (2000): 2257-2259.
Abstract;
PDF
Steric
bulk at cycloartenol synthase position 481 influences cyclization
and deprotonation.
S. P. T. Matsuda, L. B. Darr, E. A. Hart, J. B. R. Herrera, K.
E. McCann, M. M. Meyer, J. Pang, H. G. Schepmann, and W. K. Wilson. Org. Lett., 2 (2000): 2261-2263.
Abstract;
PDF
Oxidosqualene
cyclase residues that promote formation of cycloartenol, lanosterol,
and parkeol.
M.M. Meyer, M.J.R. Segura, W.K.Wilson, and S.P.T. Matsuda. Angew. Chem., 39 (2000): 4090-4092.
PDF
Directed
evolution to investigate steric control of enzymatic oxidosqualene
cyclization. An isoleucine to valine mutation in cycloartenol
synthase allows lanosterol and parkeol biosynthesis.
E. A. Hart, L. Hua, L. B. Darr, W. K. Wilson, J. Pang, and S.
P. T. Matsuda. J. Am. Chem. Soc., 121 (1999): 9887-9888.
PDF
Cloning
and characterization of the Arabidopsis thaliana lupeol
synthase gene.
Herrera, J.B.R., Bartel, B., Wilson, W.K., and Matsuda, S.P.T.
(1998) Phytochemistry 49, 1905-1911.
Abstract;full
text; PDF
Isolation
of an Arabidopsis thaliana gene encoding cycloartenol synthase
by functional expression in a yeast mutant lacking lanosterol
synthase by the use of a chromatographic screen.
Corey, E.J., Matsuda, S.P.T., and Bartel, B. (1993) Proc. Natl.
Acad. Sci. USA 90, 11628-11632.
Abstract;
PDF