
Welcome page
Timetable
Background
Methods
- Rules
- Media/culture
- Aseptic technique
- Staining/interpretation
- Isolation strategies
- Assays/characterization
- Microscopy
- Cultural characteristics
Media preparation
and training
Strategies for obtaining pure isolates
Bergey's Manual correctly stresses the importance of obtaining a pure isolate before attempting to characterize a species. The presence of just one contaminant in an otherwise homogeneous culture can lead to a misleading assay result, if the contaminant grows at a significant rate and responds differently to assay conditions. A few bacteria are morphologically unique and can be identified without isolation, but nearly all require isolation and characterization by various assay methods. Here is a description of the approach used in our teaching lab to the isolation of pure cultures from a mixture of species.
Dilution streaking and differential incubations
Sterile equipment must be used when sampling, of course. A specimen should be plated out as soon as possible after sampling. If a specimen is removed from a specialized environment such as bodily fluids, it should be cultured immediately. A sterile solution of 1% peptone can be used as a vehicle for samples to be spread on agar plates. Any liquid sample must be thoroughly vortexed prior to preparation of plates. Non-motile bacteria may settle to the bottom of a sample, particularly if they are associated with particulate matter. Bacteria do not segregate homogeneously, that is, replicate samples from the same mixture may contain vastly different quantities of bacteria. For example, in water analysis several dilute samples from a source may contain zero viable bacteria, while another may contain enough to declare the source contaminated.
Each viable cell or cluster of viable cells is called a colony forming unit (CFU). The object of thorough vortexing and subsequent dilution streaking is to spread out individual CFUs so as to obtain discrete colonies that may be subcultured. Please keep in mind that a single colony may have been derived from a CFU containing more than one viable species.
To prepare a three way dilution streak plate, a loopful of thoroughly vortexed sample is obtained aseptically and applied to one edge of the agar surface. With back and forth movements about 1/4 of the surface should be streaked while drawing the loop toward the middle of the plate. Streaking should not break the surface of the agar, and there should be many (20 or more) streak lines produced. To dilute the sample the loop must be flamed to destroy all viable material, touched to a clean part of the agar surface to cool it, then streaks made perpendicular to the original inoculum, overlapping that part of the plate once or twice. The second section should cover 1/2 of the remaining sterile surface. This spreads out a small part of the original inoculum, possibly diluting it sufficiently to result in the appearance of individual colonies after incubation. A third section is then streaked perpendicular to the second section,, flaming and cooling the loop and overlapping the previous section as before, to further dilute the inoculum.
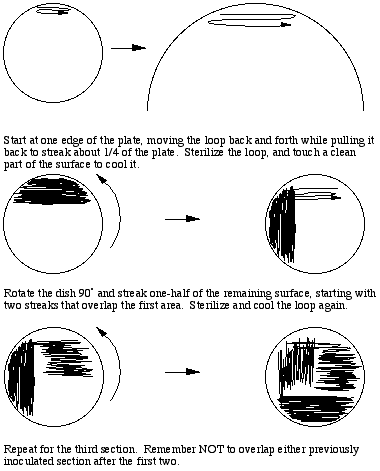
To prepare isolates from a mixed culture we typically prepare replicate streak plates and incubate them under different conditions in an inverted position, to maximize opportunities to differentiate colony types. We vary both temperature of incubation (typically 25, 30, and 37C) and incubate under both aerobic and anaerobic conditions. This approach increases the chances of separating individual species, since different species often have different optimum temperature ranges for growth, and different requirements for oxygen. Aerobically incubated plates should be checked after one day of incubation, since some species may grow very fast and crowd out the others. Any plate should be removed from the incubator after two days and examined for the presence of distinctive colonies. Leaving plates in an incubator only dries them out and clutters up the place.
Some species, such as those of genus Micrococcus, are slow to develop recognizable colonies and may require longer incubation times. You may wish to keep one or more plates for several more days to watch for the appearance of novel colonies. Keep such dishes sealed with Parafilm, and be mindful that contaminants can also show up in older plates (especially near the edges).
How to mess up a streak plate
- Start with a wet plate with liquid on the surface - the liquid will pick up material as the plate is handled, and re-inoculate the plate, making a mess.
- If you start with a liquid culture or sample, dip the loop to take an inoculum without vortexing to make the sample homogeneous.
- Forget to cool the loop before taking an inoculum - you¹ll kill the culture, and nothing will grow.
- Forget to sterilize the loop with a flame before streaking the second and/or third time. You¹ll get luxuriant growth all over the plate, and no individual colonies.
- Make only a few passes with the loop each time you streak. You can write your name with the inoculum, in fact, but you won't get individual colonies.
- Contaminate the plate by taking off the lid completely and/or sneezing on it.
- Contaminate the plate by sticking your face close to it to see the streaked area.
Approaches to identifying and separating colony types
It is important to check the cultures in a timely manner in case one or more species grows so rapidly that it will overwhelm the rest. We typically use a dissecting microscope with transilluminator to distinguish individual colonies. Plates should remain inverted during examination. Colonies should be distinguishable by size, shape, opacity, and texture of colonies. You can indicate colonies to be sampled by putting a small mark next to them on the bottom of the plate. You will have to turn the plate lid up to collect colony material. ***Open lids only sufficiently to introduce a sterile loop or needle, and only for the time it takes to obtain an inoculum.***
For color,surface characteristics, and profile (raised, flat, etc.), you will need to examine colonies with incident light, through the transparent lid. Lids should be left on, otherwise it is a near certainty that the plates will become contaminated. Before turning a plate over make sure that liquid condensate will not land on the agar surface. If necessary, "tap off" excess water by keeping the plate inverted, quickly removing and inverting the lid, and tapping it on a bench top. Alternatively, use a lid from a new plate to replace the old lid for viewing. Never leave a lid off with agar surface exposed, unless you want to add contaminating species from dust particles in the laboratory to your collection of isolates.
If two colonies overlap and still can be distinguished, then at least two species are present. Colonies usually have a fairly simple, uniform texture. If an area resembles a mosaic, you probably have at least two species. Each unique type of colony should be sampled by taking a needle inoculum and performing a three way dilution streak on a fresh plate. Care must be taken to sample only from the colony of interest. Not much material will be required. Incubate each new streak plate under aerobic conditions at the temperature at which the original plate was incubated.
Keep a record of the source of the isolate, the original incubation temperature of the plate from which the colony was sampled, and whether or not the plate was incubated anaerobically. Species will exhibit temperature optima, indicated by faster growth and/or larger colonies at a temperature closest to ideal. Any colony that is sampled from an anaerobically incubated plate will likely be a facultative anaerobe.
Purifying the isolates
Selection of a single colony from a plate does not ensure that it is pure, since non-growing or slow-growing contaminants may be present within the colony. Thus the second generation plates must be carefully screened for contaminants. Generally, all colonies on a plate containing a pure isolate will be identical in morphology, and cells will be morphologically similar in stains or wet mounts. However, there are several pitfalls of which a student must be aware.
- Bacteria with spreading growth may carry contaminants that are difficult to separate in one generation
- Spores may be misinterpreted as contaminating cell types
- The morphology of older colonies may change, leading one to believe that there are two strains
- Older Gram positive cells may lose the gram positive property
- A single species may have variants that appear morphologically different under the same culture conditions
- Contaminants from outside the plate may form viable colonies. Usually these are distinguisable because they are found only on the edge of a plate and are often not on the track of the inoculating loop.
Narrow down the collection
You are almost certain to collect duplicates of the same species and strain from different streak plates. Many different species and different strains of the same species produce very similar colony types. To narrow the number of isolates to unique species/strains, culture suspected dupicate isolates on the same plate. On two thirds of the surface conduct two "mini-dilutions" to obtain individual colonies for each culture, and on the remaining 1/3 mix them. Incubate, and if you can distinguish the two isolates grown on the same plate and/or the mixed inoculum gives you two distinguishable colony types, then you have two unique isolates. If not, then you have less work to do!
Initial characterization of colonies and preservation of isolates
A common descriptive terminology should be applied to avoid confusion when isolating and characterizing pure isolates. Since many different authors contributed to the Bergey's Manuals the student should be prepared to encounter inconsistencies among descriptive characteristics. For example, what is described as a yellow colony may actually look brown to the student. "White" usually means bone-white, but not always. In fact, many of the descriptive characters are subject to interpretation. Most characteristics, and especially color, should be determined using incident, not transmitted, light. The descriptive terms in this course will be kept relatively simple, although some of the literature makes finer distinctions among forms.
Once an isolate is obtained and purity established by both colony examination and microscopic examination, an agar slant tube should be inoculated, labeled, and incubated at an appropriate temperature with the cap loose to allow gas exchange. After luxurient growth appears, the culture should be described, the cap tightened, and the tube kept at room temperature as a source of pure culture for assays.
In addition to the gross descriptive characterization, a young (<18 h) culture should be Gram stained and results recorded including cell shape and size, sheaths or capsules if evident, and any evidence of spores or similar structures. Relationship to oxygen is the logical next step with which to narrow possible categories. After that, it is the particular combination of Gram stain result, cell type, and relationship to oxygen that determines the next series of steps toward characterizing your isolate.
Descriptive terms for colonies on agar plates
If colonies are present, describe the amount of growth as slight, moderate, or abundant. Note if colonies fail to grow under certain conditions (e.g., certain temperatures). Describe the color and whether or not the colony is opaque, transparent, or translucent. Note whether or not pigmentation has diffused into the medium itself. Describe the general colony appearance, margin, and elevation of the colony.
Growth on agar slants
When a loopful of material is used to inoculate an agar slant, the loop is touched to the surface near the bottom and drawn up toward the top to make a single broad streak. The shape of the growth that results should be described in specific terms.
Growth in broth cultures
Describe the amount of growth as for agar plates. Describe the broth beneath the surface as turbid if it is cloudy, flocculent if large flaky masses are floating in the medium. If there is sediment, note that fact, agitate the tube to bring it into suspension, and describe the sediment by size of particle and whether or not it is viscous (use a sterile loop to determine whether or not it is "gooey."
The surface may be characterized by a coating consisting of a thin membrane or a thick pellicle. There may also be a ring of material (like a pellicle or membrane, but only at the edges), or flocculent material at the top. Note that conditions in an undisturbed broth tube become anaerobic below the top 0.5 cm or so of liquid. Presence/absence of growth in deeper layers may confirm the relationship to oxygen.
Created by David R. Caprette (caprette@rice.edu), Rice University 24 May 00
Updated 18 May 15
Copyright and Intended Use