Improving the Synthesis Chemistry and Capability of Quantum Dots.

Fluorescing suspensions of CdSe quantum dots (QDs) synthesized in a heat transfer fluid, of varying particle diameters: blue (2.0 nm), green (2.5 nm), yellow (3.0 nm), orange (3.9 nm), and red (4.2 nm). This picture of CdSe quantum dot suspensions from the Group is featured in a textbook (Chemistry, 5th Edition, McCurry and Fay)
Fluorescent semiconductor NPs, or quantum dots (QDs), have potential uses as an optical material, in which the optoelectronic properties can be tuned precisely by particle size. Advances in chemical synthesis have led to improvements in size and shape control, cost, and safety. A limiting step in large-scale production is identified to be the raw materials cost, in which a common synthesis solvent, octadecene, accounts for most of the materials cost in a batch of CdSe quantum dots. Thus, less expensive solvents are needed. Heat transfer (HT) fluids is a class of organic liquids commonly used in chemical process industries to transport heat between unit operations, and have physical properties that suggest possible use as such alternative solvents for QD synthesis.
We found that two heat transfer fluids (Dowtherm A and Therminol 66) can be used successfully in the synthesis of CdSe quantum dots with uniform particle sizes. The synthesis chemistry for CdSe/CdS core/shell quantum dots and CdSe quantum rods can also be performed in HT fluids. We noted subtle differences in the growth patterns of the QDs using the different HT fluids, octadecene, and trioctylphosphine oxide. With the aid of a population balance model developed by Prof. Mantzaris, we concluded that these differences are due to solvent effects, specifically solvent viscosity, CdSe bulk solubility in the solvent, and surface free energy of the oleic-acid -covered QDs.
These findings on HT fluids use and QD growth kinetics should be applicable to NPs of other compositions, and can help guide the development of control strategies for liquid-phase production of high-quality NPs.
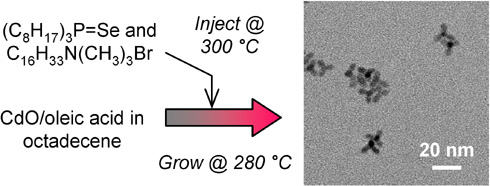
Schematic of new synthesis method for CdSe tetrapods

Collage of transmission electron micrograph (TEM) images, of uniform CdSe tetrapods of various sizes. Images are false-colored to indicate
the emitted color of the tetrapods. Scale bar = 50 nm.
Along the same lines, the synthesis of 4-legged CdSe QDs called tetrapods are difficult to scale up. They are difficult to prepare with uniform arm lengths and widths; non-tetrapod QDs invariably get formed simultaneously; and the synthesis requires expensive and toxic phosphonic acid surfactants. We discovered that cationic ligands in the form of quaternary ammonium surfactants induces faceting in QDs, and that cetyltrimethylammonium bromide (CTAB) surfactant in particular lead to ~90% selective synthesis of uniform CdSe tetrapods. The use of CTAB enables the greener and scalable synthesis of tetrapods, which are currently being studied for solar cell applications.
Selected Publications
W. Y. L. Ko, H. G. Bagaria, S. Asokan, K.-J. Lin and M. S. Wong, "CdSe Tetrapod Synthesis Using Cetyltrimethylammonium Bromide and Heat Transfer Fluids," J. Mater. Chem. 20 (12), 2474-2478 (2010). DOI:10.1039/b922145j (Abstract)
S. Asokan, K. M. Krueger, V. L. Colvin, and M.S. Wong, "Shape-Controlled Synthesis of CdSe Tetrapods Using Cationic Surfactant Ligands", Small 3(7), 1164-1169 (2007). DOI:10.1002/smll.200700120 (Abstract)
S. Asokan, K. M. Krueger, A. Alkhawaldeh, A. R. Carreon, Z. Mu, V. L. Colvin, N. V. Mantzaris and M. S. Wong, "The Use of Heat Transfer Fluids in the Synthesis of High-quality CdSe Quantum Dots, Core/Shell Quantum Dots, and Quantum Rods", Nanotechnology 16, 2000-2011 (2005). DOI:10.1088/0957-4484/16/10/004 (Abstract)