
Welcome page
Timetable
Background
Methods
- Rules
- Media/culture
- Aseptic technique
- Staining/interpretation
- Isolation strategies
- Assays/characterization
- Microscopy
- Cultural characteristics
Media preparation
and training
Staining and Interpretation of Smears
Important information such as shape and degree of motility can be obtained by observation of living bacteria with the phase contrast or dark field microscope. However bacteria are routinely stained with different dyes in order to reveal different properties and to enhance contrast for viewing with conventional bright field microscopy. A number of stains have been developed to distinguish spores, nuclear bodies, capsules, and characteristics of the cell wall.
The staining methods we will use kill the bacteria, reducing the risk of infection by pathogenic organisms. Since
the rigid cell walls of bacteria prevent distortion of morphology upon drying, samples can be spread onto a glass
slide and air dried, then fixed to the surface by passing the slide quickly through a flame, melting the complex
carbohydrates of the cell walls to the glass and killing the cells.
The Gram stain is routinely used as an initial procedure in the identification of an unknown bacterial species.
Bacteria bear a slight net negative charge and usually bind positively charged dyes such as methylene blue and crystal
violet. A species can be classified as Gram positive, Gram negative, or Gram variable depending on the ability if
cells to retain the blue dye. Gram negative bacteria do not retain the dark blue color, but can be counterstained
a light red so that they can be seen in bright field microscopy. Since two dyes are used to distinguish types of
bacteria, Gram staining is called a differential staining method.
The Gram stain is a direct method, since the cells themselves retain dye. In indirect, or negative, staining, smears are produced by mixing material with India ink or acidic dyes such as nigrosine. Acidic dyes have a negative charge and are repelled by the negatively charged cell walls. Cells remain unstained against a dark background.
Some species produce spores, which are dormant cells with thickened cell walls. Spores are often detectable in Gram stains or by phase contrast microscopy of living cells, however differential staining methods may be necessary to confirm or reject the presence of spores in a culture. As with the Gram stain, a spore stain distinguishes spores on the basis of cell wall properties.
In the laboratory you will practice the Gram stain technique on a variety of Gram positive and Gram negative bacteria grown on different types of media. You will learn the technique of negative staining with nigrosine dye in order to clearly distinguish shapes of bacteria. You will also carry out spore staining on appropriate cultures. Finally, you will observe living bacteria to become familiar with features that can be seen without staining, including cell shapes, patterns of association, and motility.
Preparing a smear
A properly prepared smear accomplishes two things. It causes bacteria to adhere to a slide so that they can be stained
and observed. It also kills them, rendering pathogenic bacteria safe to handle. An objective in preparing smears
is to learn to recognize the correct density of bacteria to place on the slide. Too many, and they overlap each other
giving false positives or crowding each other to make a mess. Too few, and they cannot be located on the slide.
- Use a glass etching tool to mark a circle on the under side of a slide at each positioin at which you plan to make a smear. Several circles can be located on the same slide. The circles help you locate smears that are hard to see with the naked eye and help you locate the surface of the slide on which you have made the smear.
- The slide must be grease-free. A good way to clean a slide is to repeatedly breathe on it, followed by rubbing vigorously with a Kimwipe or paper towel to remove the fog. When the slide de-fogs immediately after breathing on it, it is sufficiently clean.
- To prepare a smear from a colony, place a loopful (~25 µl) of deionized water over the circled area. Aseptically remove a barely visible amount of material from a culture with a loop or stick and use the loop or stick to mix it with the drop. Try to disperse the culture material completely, so that there are no visible chunks of material.
- To prepare a smear from a broth culture, aseptically remove several loopfuls of culture and spread over the circled area.
- Allow the drop to air dry completely (usually a couple of minutes for a single loopful).
- Light a bunsen or Fisher burner and adjust to produce a vigorous flame. Hold the slide horizontally with a clothes pin and pass it through a flame three times with the smear up, to kill the bacteria and cause them to adhere. Each pass through the flame should take about a second.
- After cooling the slide, conduct the staining procedure.
How to mess up a smear
- Forget to clean the slide
- Use too much material - before air drying the smear the liquid should be somewhat cloudy and without particulate matter
- Use so much liquid that it takes forever to dry
- Heat the smear before letting it air dry, boiling the bacteria instead of attaching them
- Overheat the smear, melting cell walls and possibly breaking the slide
The Gram stain
In Gram staining bacteria fixed to a slide are treated with a basic dye that binds electrostatically to the negatively charged cells. Next, the preparation is treated with a mordant such as iodine to form an insoluble dye-iodine complex. The slide is then washed with alcohol to solubilize and remove the dye-mordant from Gram negative cells but not Gram positive ones. Differential extraction of the dye-mordant by the decolorizing agent is the critical step that distinguishes the bacteria. A counterstain, safranin, is applied in the final step. Cells that have been decolorized will take up the second basic dye whereas those already stained with the first dye will not.
The mechanism of the differential staining response has not been resolved with certainty. One theory holds that differences in the cell wall chemical composition account for the staining response. A second theory maintains that the thicker walls of Gram positive bacteria are dehydrated by the decolorizing solution and shrink, resulting in the closure of pores in the wall, trapping the dye-mordant within the cell. The thinner cell wall of Gram negative bacteria would be readily penetrated by the decolorizer. What is known with certainty is the critical role of the cell wall. Removal or alterations of the wall from Gram positive organisms converts them to Gram negative cells.
Since many Gram positive bacteria tend to become Gram negative with age, the Gram stain should be used with overnight cultures. Sample from the edge of a colony, where cells are actively growing.
You may want to wear hand protection and clothing that you don't care about too much. The chemicals are not particularly toxic but they will stain skin and clothing. Stains on the skin typically wear off in less than a day, but stains on clothing are usually permanent.
Components
Different formulas have been used for crystal violet, safranin, and decolorizer, all of which are effective. In the teaching laboratory these solutions will be provided ready made.
- Gram's crystal violet: 1% aqueous crystal violet dye; [Hucker's crystal violet] 2 g crystal violet 90% dye content, 20 ml ethyl alcohol, 0.8 g ammonium oxalate, 80 ml distilled water
- Gram's iodine: 1 g iodine, 2 g potassium iodide, 300 ml distilled water
- Gram's safranin: 4 g safranin powder, 200 ml ahydrous ethanol, 800 ml distilled water
- Gram's decolorizer: 25% acetone, 75% isopropyl alcohol
Leaving a slide covered with stain for longer than the recommended times does no harm, provided the stain does not dry up. There is no benefit either.
- Place the slide with smear(s)s up on a rack in a staining tray. You may find it convenient to leave the clothes pin attached. Flood the slide with Gram's crystal violet so that all of the dried material is covered. It is good practice to flood the entire slide from edge to edge.
- After 60 sec pick up the slide using the clothes pin and rinse the smear with water by squirting the slide above the smear and letting the water wash over it until the water runs clear. To keep the slide "clean" you might rinse the bottom as well, and make sure you have not trapped stain underneath the clothes pin where you have grabbed the slide. Note that the smears will be stained a bright blue.
- Flood the slide with Gram's iodine (the mordant), leave for 60 sec., then pick up the slide and rinse again with water as described above. The smears should have turned nearly black. If not, then the iodine may have gone bad or you applied it to the wrong side of the slide.
- Following the water rinse, use a gentle stream from a pasteur pipet to rinse the smears with Gram's decolorizer. Color should come off at first. Continue rinsing until the wash is colorless, then rinse again with water.
- Flood the slide with Gram's safranin for 60 sec. followed again by a water rinse. At this point, smears of Gram positive cultures should be stained nearly black, while smears of Gram negative cultures should stain pink to red. To be sure, though, you must observe them in the microscope.
- Blot the smear to remove excess water, using bibulous (absorbent) paper or a paper towel. Do not wipe the smears or you may wipe them off. Allow to air dry.
How to mess up a Gram stain
- Mess up the smear to start with
- Apply stain to the wrong side of the slide
- Stain or rinse the smear incompletely
- Allow stain to dry up before rinsing
- Forget to decolorize or decolorize incompletely
- Rub off the material when you blot it
Observing a Gram stain in the microscope
We employ bright field microscopy for observing Gram stains. A good approach to observing any smear of very minute objects is to examine it under low power (40x final magnification) to become oriented. After focusing, one then works up to 100x, 400x, and finally oil immersion at high power (usually 1000x). The immersion oil is placed directly on the smear. By the way, the focal lengths of the high dry and oil immersion lenses are less than the thickness of a slide. The smear must be up, or focusing won't be possible. The top of the slide can be identified by feeling for the etched circle on the bottom of the slide.
At low magnifications Gram stained material looks like dirt on the slide. Higher magnifications are needed in order to see any detail at all. Bacteria are often concentrated in a ring around the original smear. Bright field oil immersion microscopy is necessary to see an undistorted image of any directly stained bacterial smear. A Gram negative or positive phenotype cannot be confirmed with certainty using only a dry magnification, since typical cells are a half micrometer in diameter, less than the best resolution of the high dry lens. Phase contrast or dark field viewing improve the resolution, but both distort the color. High dry magnification distorts both shape and color.
After putting immersion oil on a slide, the high dry (40x) lens can't be used again unless the oil is removed. Slides are usually blotted to remove excess oil, then dipped in xylene several times to dissolve the oil film, and air dried in the fume hood.
Indirect (negative) staining
The acidic dye nigrosine can be used to visualize the capsule or sheath that surrounds some bacteria in a process called negative staining. Capsules are composed primarily of polysaccharides or glycoproteins and are gelatinous in texture. They are readily destroyed by heating and hence direct staining methods cannot be utilized. In general, the size and shape of microorganisms is often less distorted with indirect staining procedures, especially when sampled from a broth culture. Therefore negative staining is useful whenever the morphology of individual bacteria is in question. Morphology can often be determined with confidence with only the high dry lens. Consider that this procedure does not necessarily kill the organism, so be careful.
- After preparing a clean, greaseless slide, a small drop of nigrosine is mixed with a small drop from a broth culture or with a quantity of dry material.
- The drop is spread across the slide using the edge of another slide as a spreader. This same procedure is used for blood smears.
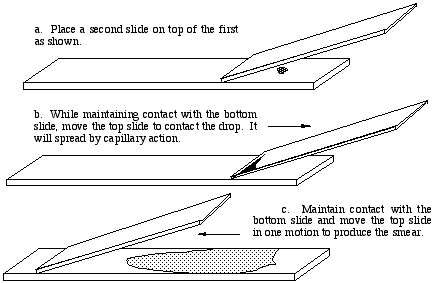
After air drying, the smear is observed using the high dry lens, or oil immersion if desired. The smear will be most dense
where the nigrosine dye was deposited on the slide. Somewhere along the tapered spear the density will be ideal. The
background should be blue-gray. Bacteria will be evident by the absence of any color.
Spore staining
The following modification of the Wirtz method, described below, has been effective and trouble free in our experience. You are cautioned, though, that the color may fade after a few days. The result is a delicate combination of green and red that is readily recognized provided lighting is optimum. ***CAUTION*** Malachite green dye is toxic, and breathing of fumes or contact with skin can be hazardous. This procedure should be carried out in a fume hood. The stand, burner, dyes and pipets will be available in the hoods. The procedure is messy - wear a lab coat or old clothes.- A smear is prepared and fixed with 20 passes through a flame.
- The smear is placed on a screen well above a moderate flame from a bunsen burner.
- A generous amount of saturated aqueous malachite green is applied to the slide and allowed to steam off for 10 to 20 minutes. Ideally, the heat is maintained so that the dye barely steams, that is, whitish vapors are barely visible. Dye must be added so that the liquid does not dry up.
- After cooling the slide is rinsed with tap water to remove excess stain.
- The slide is then counterstained for a minute in 0.25% aqueous safranin. The slide is then rinsed, blotted, and dried.
Spores stain a light green,while the rest of the cell stains pink. Spores are best seen with oil immersion microscopy. Often, the colors are not very strong, so it is necessary to have the microscope in good alignment with optimum contrast and lighting. Make color notes right away, as the green may fade after a few days.
Observations on living bacteria
Sometimes assay results are compromised because a contaminating organism grows in the medium instead of the intended bacterial isolate. For a quick check to verify that cell morphology is consistent with the culture from which the inoculum was taken, a wet mount can be prepared and examined in dark field and/or phase contrast. If present, endospores are often evident in phase contrast, allowing one to avoid having to do a spore stain.
Very often, identification of an unknown organism requires knowledge of its motility, that is, its capability for translational movement. The results of motility agar incubations can be difficult to interpret, partically for aerobic bacteria that don't grow well deep into the agar. A good quick check for motility is to examine a very young culture using the hanging drop method. A young culture would be a broth culture inoculated the night before, or a broth culture that was diluted 10 fold or so in the morning, incubated, and examined in the afternoon. A hanging drop culture is prepared by placing a very small drop of medium on a coverslip, then inverting the coverslip over a depression slide so that the bottom of the drop does not make contact with the slide itself. Vaseline can be used if necessary, to make a sealed chamber.
Hanging drops can be examined using all objective lenses, although to be able to look throughout the depth of the drop the limit may be 100x. The curved depression slide will distort the effects of phase contrast, but dark field may work and will be sufficient to detect movement. All live bacteria move by Brownian (molecular) motion, at a vibration rate that is inversely proportional to the size of the cell. Rapid Brownian movement is a common characteristic of non-motile cocci such as Staphylococcus, Streptococcus, or Micrococcus. However some bacteria are flagellated, and exhibit translational movement as well. Truly motile organisms will zip across the microscope field. Look for definite directional motion, tumbling, and movement against currents.
Dispose of wet mounts carefully, since the bacteria will be viable.
Created by David R. Caprette (caprette@rice.edu), Rice University 24 May 00
Updated 17 Jul 14
Copyright and Intended Use