Chapter 3 - Conservation of
Mass
OVERVIEW
Conservation of mass is the topic of Chapter 3.† The challenge problem is tissue engineering
and its application for bone enhancement and replacement;† worked example problems include flow through
a bone graft, oxygen consumption in bone, and toxin accumulation in a
laboratory bone implant.† The principles
of mass balances are illustrated first for open, non-reacting, steady-state
systems.† Systems with multiple inlets
and outlets and then systems with multicomponent mixtures are considered.† More complex multiple-unit systems are
illustrated by a two-compartment model of the kidney and by a wastewater
treatment facility.† Systems with chemical
reactions, such as respiration, are explicitly covered.† Terms such as reaction rate and fractional
conversion are defined.† Finally, dynamic
systems such as drug delivery are addressed.†
EXAMPLE PROBLEMS
1.† The
drug streptomycin is produced on a large scale in the U.S.† After purification, streptomycin is 50 wt% in
water.† For applications, including I.V.
drips, the streptomycin must be diluted and preservative must be added.† The diluent stream contains 2 wt% NaCl in
water.† The preservative stream contains
10 wt% preservative and 5 wt% NaCl in water.†
These three streams are mixed together in a mixing tank.† The outlet stream is ready for packaging in
I.V. bags.†
a. Determine the ratio of the stream containing the drug to the outlet
stream given that the drug is 10 wt% in the outlet stream.
b. Determine the ratio of the stream containing the preservative to the
outlet stream given that the preservative is 3 wt% in the outlet stream.
c. Determine the ratio of the diluent stream to the outlet stream given
that the drug is 10 wt% and the preservative is 3 wt% in the outlet
stream.† (You answer may include an
unknown variable.)
2. † During cellular metabolism, glucose is combusted
to carbon dioxide and water.† One of the
many steps in glycolysis is the Krebís cycle.†
A biochemical summary of several steps in the Krebís cycle is as
follows:
††††† 1 C6H8O7
(citric acid) + a H2O ŗ p C4H4O5
(oxaloacetic acid) + q H + r CO2
††††† It is known
through biochemical experiments that for every molecule of citric acid
consumed, one molecule of oxaloacetic acid is generated.†
a. Balance the above equation.†
Determine the stoichiometric coefficients a, p, q, and r.
A tissue mass
is comprised of many cells, each which conducts the process of glycolysis
including the Krebís cycle.† Assume a
molar flow rate of 0.1 mol/day of C6H8O7 into
this tissue.
b. What is the minimum flow rate of water needed for a fractional
conversion of the C6H8O7 to be 1.0?
c. Assume that the fractional conversion of water is 0.8 and the
fractional conversion of C6H8O7 is 1.0.† What is the limiting reactant?† Calculate the reaction rate (R) and the inlet
molar flow rate of H2O.†
Determine the molar flow rates of the products and the excess reactant
leaving the tissue.
3.† Biodegradable synthetic materials are now being
explored for use as carriers for drug delivery.†
Poly(lactic-co-glycolic) acid (PLGA) is one such material currently
being explored for this purpose as it is already approved by the FDA for use in
the human body.† Microspheres loaded with
drug can be fabricated.† By varying the
properties of the polymer comprising the microsphere, the release profile of
the drug can be altered systematically.
††††† You have run an experiment to determine
the effects of microsphere diameter on the release of the model drug, FITC-BSA
(fluorescently-labeled bovine serum albumin).†
The release curve for spheres (diameter = 30 mm) is shown in Fig.
3P.12.† After fitting a curve to your
data, you find that the release of FITC-BSA can be modeled as follows:
††††††††††††††††††††††† 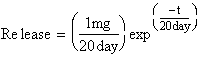
††††† Initially, the
mass of the model drug FITC-BSA in the microsphere is 1 mg.† Determine the amount of drug released after
30 days.
Chapter 2 - Foundations of Conservation Principles | Chapter 4 - Conservation of Energy