2025 Abstracts.
207. Hong,K., Elias, W., Heck,K., Terlier, T., Dimpel, D., Qian, W., Liu, B., Kurihara, T., Wong, M.S. "Pulsed laser ablation-synthesized FePt nanoparticles have enhanced catalytic nitrite reduction activity" Catalysis Today 2025, 453, 115254 DOI: https://doi.org/10.1016/j.cattod.2025.115254
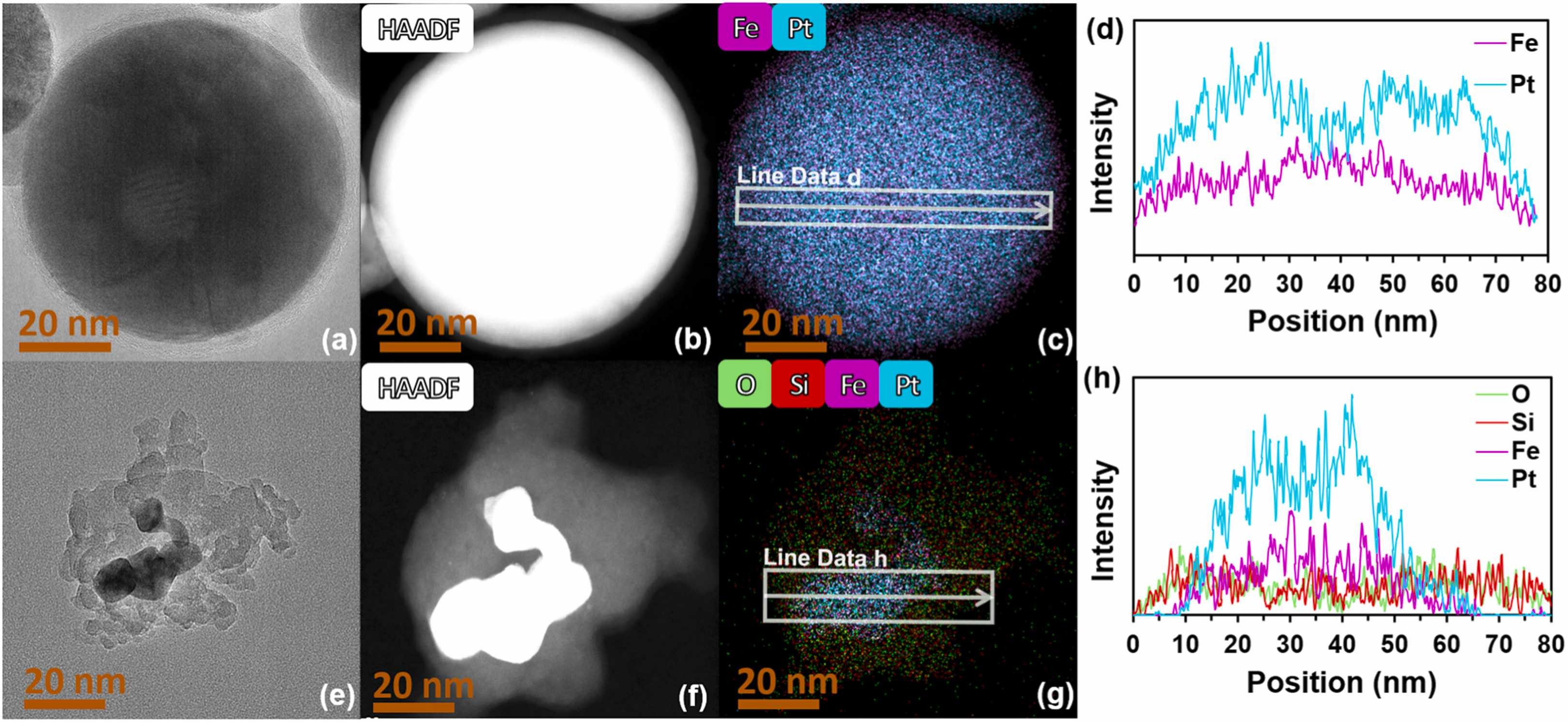
Nitrate/nitrite pollution of water resources is an ubiquitous problem primarily due to the over application of fertilizer. While commercial methods which remove nitrates/nitrites exist, techniques which directly destroy the pollutants are more desirable. Nitrate/nitrite reduction by precious metal catalysts is one promising method to permanently convert nitrate/nitrite to non-toxic analogs. Amongst these, iron-platinum (“FePt”) compositions are generally considered inactive for NO2-. In this study, we demonstrate that bimetallic FePt catalysts synthesized via a pulsed-laser-ablation-in-liquid method (“PLAL-FePt NPs”) exhibit notably improved activity for NO2- compared to those synthesized by incipient wetness impregnation (“FePt/SiO2”) with the same elemental composition. While FePt/SiO2 catalyst (60 at% Pt) had nitrite reduction activity, the PLAL-FePt NPs (57 at%) were ∼8 times more active (kcat ≈ 4.6 vs. 39.5 Lmin-1gsurface Pt-1) on a per surface Pt basis. When compared to the monometallic Pt catalyst, the PLAL-FePt NPs were ∼2 times more active. X-ray diffraction (XRD) show PLAL-FePt NPs to have intermetallic phases of Pt3Fe1 and Pt1Fe1. In contrast, FePt/SiO2 have multiple phases (Pt, Pt3Fe1, Pt1Fe1, and Pt1Fe3). X-ray photoelectron spectroscopy (XPS) showed that Pt gained electron density from Fe, correlating to the increased nitrite reduction activity of both bimetallic materials. These findings indicate an earth-abundant element like iron can improve platinum catalysis, highlighting Fe-Pt intermetallic alloys for further study as hydrogenation catalysts.
209. Glass, S., Kannan, H., Bangala, J., Chen, Y., Metz, J., Mowzoon‑Mogharrabi, R., Gao, G., Meiyazhagan, A.K., Wong, M.S., Ajayan, P.M., Senftle, T.P., Alvarez, P.J.J. "Iron Doping of hBN Enhances the Photocatalytic Oxidative Defluorination of Perfluorooctanoic Acid" ACS Applied Materials & Interfaces 2025 DOI: https://doi.org/10.1021/acsami.5c01963
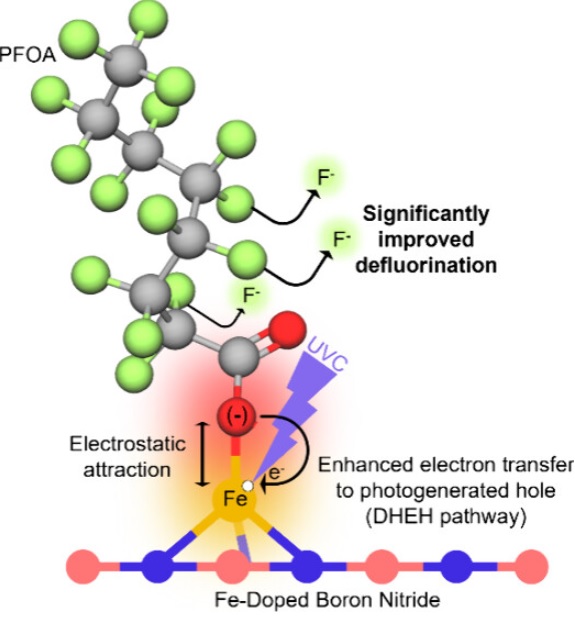
There is a growing need to effectively eliminate perfluorooctanoic acid (PFOA) from contaminated water, which requires extensive defluorination. Photocatalysis offers potential for PFOA degradation under ambient conditions without the need for treatment chemicals. However, photocatalytic treatment generally results in limited defluorination and, thus, incomplete elimination of potential toxicity and liability. This underscores the need to advance mechanistic understanding of the factors limiting PFOA oxidative defluorination. Here, we tested the hypothesis that direct electron transfer from PFOA to transition metals enhances photocatalytic defluorination. We developed a novel, facile approach to simultaneously functionalize and dope hexagonal boron nitride (hBN) (which is known to effectively catalyze photocatalytic PFOA oxidation) with Fe(III), using deep-eutectic solvents (DES). Addition of Fe(III) to synthesize Fe-hBN created new active sites for PFOA oxidation and doubled the defluorination extent (>40% fluoride release from initial 50 mg L–1 PFOA) compared to undoped hBN in 4 h reactions under 254 nm irradiation (64.4 W m–2). The mechanism of defluorination was elucidated through scavenger experiments that show the importance of photocatalytically generated electron holes for initiating PFOA degradation. Experiments also suggest that Fe(III) played a key role in PFOA removal, contributing to the improved extent of defluorination over undoped hBN. Density functional theory indicates that Fe(III) sites enable electrostatic adsorption of PFOA to the catalyst surface, enhance charge transfer, and promote hole localization to improve charge carrier separation, which is essential for oxidative defluorination of PFOA. This mechanistic insight informs catalytic material design to enhance oxidative defluorination processes.