2017 Abstracts.
119.W. Zhou, N. Soultanidis, H. Xu, M.S. Wong, M. Neurock, C.J. Kiely, and I.E. Wachs, "Nature of Catalytically Active Sites in the Supported WO3/ZrO2 Solid Acid System: A Current Perspective" ACS Catalysis, 7(3), 2181-2198 (2017) DOI:10.1021/acscatal.6b03697
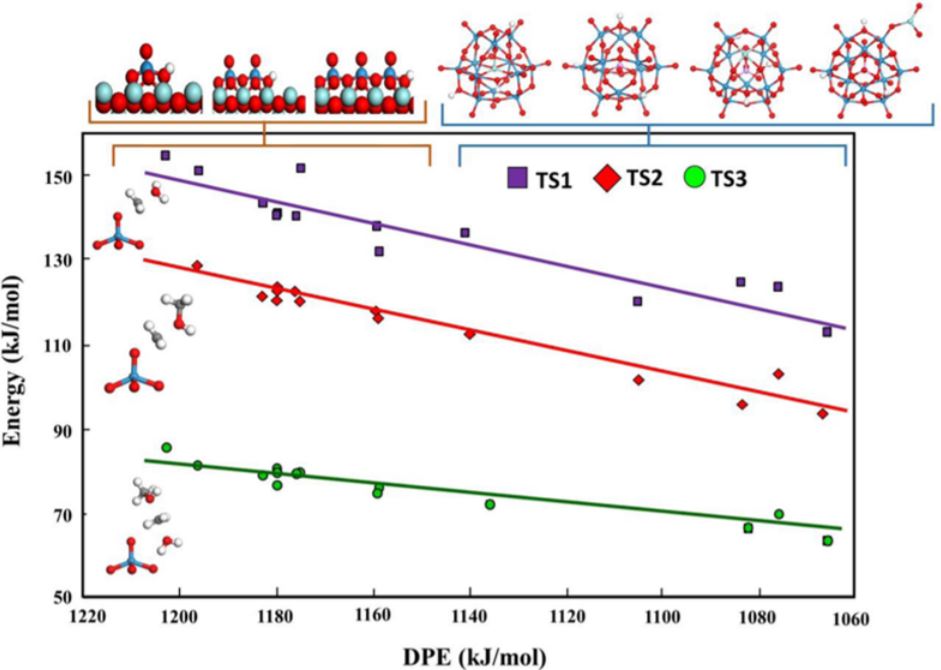
Tungstated zirconia (WO3/ZrO2) is one of the most well-studied solid acid catalyst systems and continues to attract the attention of both academia and industry. Understanding and controlling the properties of WO3/ZrO2 catalysts has been a topic of considerable interest over almost the past three decades, with a particular focus on discovering the relationship between catalytic activity and the molecular structure of the surface acid site. Amorphous tungsten oxide (WOx) species on ZrO2 surfaces were previously proposed to be very active for different acidic reactions such as alcohol dehydration and alkane isomerization. Recent developments in electron optical characterization and in situ spectroscopy techniques have allowed researchers to isolate the size, structure, and composition of the most active catalytic species, which are shown to be three-dimensional distorted Zr-WOx clusters (0.8-1.0 nm). Complementary theoretical calculations of the Bronsted acidity of these Zr-WOx clusters have confirmed that they possess the lowest deprotonation energy values. This new insight provides a foundation for the future characterization and theory of acidic supported metal oxide catalytic materials that will, hopefully, lead to the design of more active and selective catalysts. This perspective presents an up-to-date, comprehensive summary of the leading models of WO3/ZrO2 solid acid catalysts.