2015 Abstracts.
112. L. A. Pretzer, K. N. Heck, S. S. Kim, Y. Fang, Z. Zhao, N. Guo, T. Wu, J. T. Miller, M. S. Wong, "Improving gold catalysis of nitroarene reduction with surface Pd" Catalysis Today, in press (2015)DOI: 10.1016/j.cattod.2015.07.040
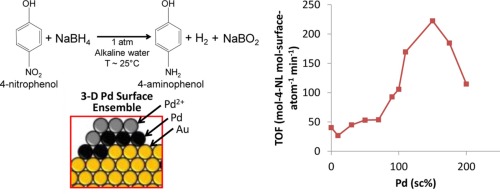
Nitroarene reduction reactions are commercialized catalytic processes that play a key role in the synthesis of many products including medicines, rubbers, dyes, and herbicides. Whereas bimetallic compositions have been studied, a better understanding of the bimetallic structure effects may lead to improved industrial catalysts. In this work, the influence of surface palladium atoms supported on 3-nm Au nanoparticles (Pd-on-Au NPs) on catalytic activity for 4-nitrophenol reduction is explored. Batch reactor studies indicate Pd-on-Au NPs exhibit maximum catalytic activity at a Pd surface coverage of 150 sc%, with an initial turnover frequency of ~3.7 mol-nitrophenol/mol-metalsurface/s, which was ~5.5× and ~13× more active than pure Au NPs and Pd NPs, respectively. Pd NPs, Au NPs, and Pd-on-Au NPs below 175 sc% show compensation behavior. Three-dimensional Pd surface ensembles (with 4–5 atoms) previously identified through X-ray adsorption spectroscopy provide the active sites responsible for the catalytic maximum. These results demonstrate the ability to adjust systematically a structural feature (i.e., Pd surface coverage) to yield a more active material.
111. M. M. Viana, M. C. F. S. Lima, J. C. Forsythe, V. S. Gangoli, M. Cho; Y. Cheng, G. G. Silva, M. S. Wong, V. Caliman, "Facile Graphene Oxide Preparation by Microwave-Assisted Acid Method" J. Braz. Chem. Soc., 00(00), 1-7 (2015)DOI: 10.5935/0103-5053.20150062
Few-layered graphene oxide (GO) was prepared using a fast and energy-saving method by microwave-assisted acid technique. The oxygenated groups existing on the GO surface were determined using UV-Vis, X-ray photoelectron and Fourier-transform infrared spectroscopies. An oxygenated group percentage of 30% in mass for the GO was observed by thermogravimetric analysis. The reduced few-layered graphene oxide (rGO) film annealed at 110°C deposited onto a silicon/silica wafer showed expanded graphite-like structure with 0.70 nm between the rGO sheets, as determined by X-ray diffraction. This rGO film exhibited a relatively high electrical conductivity value of 7.36 × 102 S m-1 confirming the good restoration of the p-conjugated system. The prepared GO sample exhibited good stability in water from pH 4 to 12, as determined by its zeta potential, and contained 5 to 9 layers, as determined by atomic force microscopy (AFM) and transmission electron microscopy (TEM).
110. Z. Zhao, J. T. Miller, T. Wu, N. M. Schweitzer, M. S. Wong, "EXAFS Characterization of Palladium-on-Gold Catalysts Before and After Glycerol Oxidation" Topics in Catalysis, 58(4-6), 302-313 (2015)DOI: 10.1007/s11244-015-0371-3
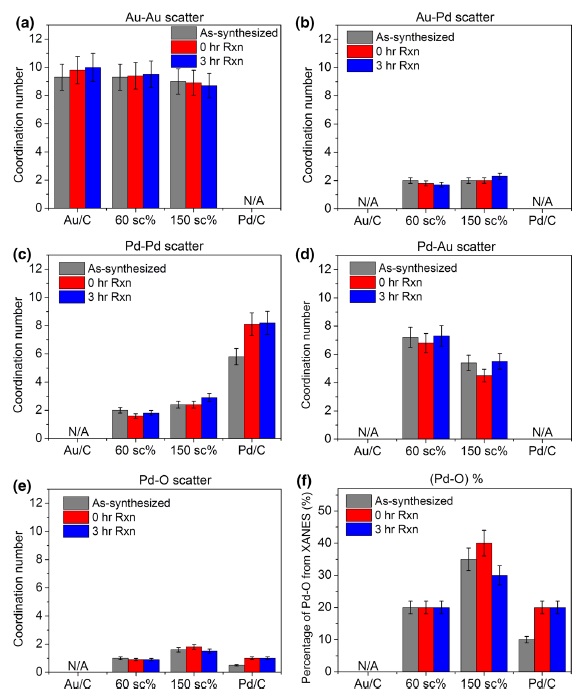
Supported precious metal catalysts have been studied extensively for selective oxidation as a means to upgrade glycerol, a low-cost byproduct of biodiesel manufacture. We recently used a model bimetallic catalyst (Au nanoparticles decorated with Pd and immobilized onto carbon, “Pd-on-Au/C”) to study the metal nanostructure effects on glycerol oxidation. In this study, a detailed X-ray absorption spectroscopy analysis of Pd-on-Au catalysts before and after glycerol oxidation (60 °C, 0.1 M glycerol, 0.4 M NaOH, and constant O2 flow at 1 atm) is presented. Catalysts with two Pd surface coverages (60 and 150 sc%) with comparable turnover frequency values were studied, along with the less active 4-nm Au/C and 4-nm Pd/C as control samples. Extended X-ray absorption fine structure analysis showed that there was no change to oxidation states and coordination numbers for 60 sc% Pd-on-Au/C and Au/C catalysts after contact with the glycerol reaction medium or after 3 h of glycerol reaction. With a higher fraction of oxidized Pd (~40 %) than 60 sc% Pd-on-Au/C (~25 %), the 150 sc% catalyst showed some variation in oxidized Pd content before and after glycerol reaction. Pd/C grew in Pd particle size and became more oxidized after contacting reaction medium and after 3 h reaction, contributing to its observed catalyst deactivation. Structural stability and catalytic activity are improved for the water-phase oxidation of glycerol and likely other alcohols when Pd is supported on Au, highlighting the potential advantages of using Au as a support for other catalytically active metals.