Arthur
A. Few Wednesday, November 12, 2003
Earth Energy Balance Models
Reference: System Behavior and System Modeling
and Training Exercise, Workshop on Modeling in the Classroom
(these notes)
By: Arthur A. Few
I think this exercise is a great learning device for
the undergraduate students. In addition to the obvious computer skills
that are learned, consider the important scientific principles involved
in this exercise and the associated reading in the text.
1. The Conservation of Energy & 1st Law of Thermodynamics
2. The Transformation of Energy: Light - Heat - Infrared
3. Heat Capacity & Temperature
4. Albedo, Solar Constant, Effective Planetary Temperatures
5. The Blackbody Radiation Law
6. Kirchoff’s Law: Absorptivity = Emissivity
7. The Greenhouse Principle
8. The Role of the Oceans in Climate
9. System Behavior: Time Constants, Feedback, Initial Conditions,
Asymptotic Approach to Equilibrium, Steady State
10. And, The Human Input of Atmospheric CO2
Part 1. Creating a Working Model with STELLA
Create your own model for the Earth energy system described in
The exercise should be done in units of years, and DT should be of the
order of 0.01 year; Euler’s method is sufficient. Using a time unit of
a year requires that we convert all physical parameters (i.e.,
Solar constant & Stefan-Boltzmann constant) from units containing
seconds to years. (One day is 86,400 s, and one average year is
31,557,600 s, which includes the leap-year effect.)
© 1992, Arthur A. Few. All
rights reserved.
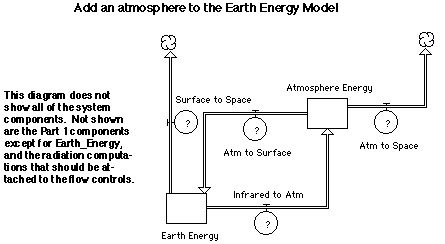
Part 2. Add
a One-Layer Atmosphere.
The basic Earth energy model created in Part 1 will be modified by
adding an atmospheric layer with radiative properties similar to our
atmosphere (i.e. transparent to visible and absorbing in the
infrared). The atmospheric parameters will be adjusted to achieve a
global temperature appropriate for the Earth.
Make a copy of your model from Part 1 (Duplicate or Save As), and
modify it by adding the Atmosphere Energy reservoir and infrared energy
flows shown below (Infrared to Space has been replaced by the
components shown below). Change the Water Depth to 100 m (the mixing
depth for the oceans). It will now have the appropriate time constant
relative to the atmosphere. This also allows for larger time steps
(~0.1 year), but requires running the model for 100 years to reach
equlibrium.

When you examine the Infrared to Space equation in Part 1 you will
notice that it is the black-body radiant flux, (area)*sT4.
The atmosphere is not a black body, but we may use a “gray body”
approximation for the atmosphere in which the radiant flux from the
atmosphere is given by (Atmos Ab Coef)*(area)*sT4. (This is
Kirchhoff’s Law.) In this expression Atmos Ab Coef is the atmospheric
absorption coefficient, a number between zero and one. The atmosphere
radiates equally upward and downward. The fraction of the Earth surface
radiation absorbed by the atmosphere is determined by Atmos Ab Coef,
and the fraction of the Earth surface radiation that passes through the
the atmosphere to space is (1 - Atmos Ab Coef).
You will also need the following information to complete the model:
The mass of the atmosphere, Mass Atmos = 5.14e18 kg.
Specific heat of air, Sp Ht Air = 1004 J/kg K.
Change the depth of the water in the Earth surface, Water Depth = 100 m.
Simulation Time settings: Experiment with these values. The model
exhibits strange behavior with dt = 1.0 years because the time step is
larger than the atmospheric time constant. Try it, and watch the
atmospheric temperature.
Computation Method = Euler’s.
The parameter “Atmos Ab Coef” is our unknown in Part 2. We know from
Part 1 that without an atmosphere the surface temperature of the Earth
is 255 K. The mean temperature of the Earth’s surface is now
approximately 288 K ~ 15 C. You should, using the technique of trial
and error correction, find the value of Atmos Ab Coef which produces an
Earth surface temperature of 288 K. Make a list of your trials and
results. Print a copy of your system diagram,
your equations, and your
graphic results to submit as part of your assignment. You may print
the
graphic results of each trial or just print your final trial on which
you write the results of your previous trials. You must submit the
answers to your trials.
You should record the values of the Earth Energy and Atmospheric
Energy from Part 2 to use as the initial values for Earth Energy and
Atmospheric Energy in Part 3; you can eliminate or reduce the warm-up
period of the Earth climate model.
Part 3. Explore the Effect of Doubling
Atmospheric CO2.
We are going to modify the model of Part 2 to explore the effect of
doubling atmospheric CO2
Add the components shown to the right to your system
diagram. Atm Ab Coef becomes a variable in Part 3.
We need to develop a relationship between a , Atm Ab
Coef, and the changing concentration of carbon dioxide, which is
controlled by the multiplier, CO2 Increase Factor, which ranges from 1
to 2. I have not found a source for a in the literature
because we are treating the whole atmosphere as a single layer. We will
develop the needed relationship from empirical data and GCM m
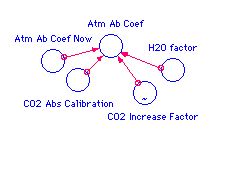
odel results. I will give you the results of my
evaluation here and provide the details in the footnote.
a0
The increase in a is a linear function of the increase
in the fractional carbon dioxide concentration
(f - 1). w is a multiplier for the
water-vapor feedback, and b is the constant of
proportionality determined by comparison with GCM model output. The
values for these parameters can be found in the footnote. You will find
that w is given over a range of values; choose a value
near the middle of this range for the first model run.
The converter – CO2 Increase Factor – is a graphic variable that
specifies the changes in CO2 Increase Factor over time. The ~ in the
converter identifies it as a graphical converter. The figure below
shows how your graphic variable should look.
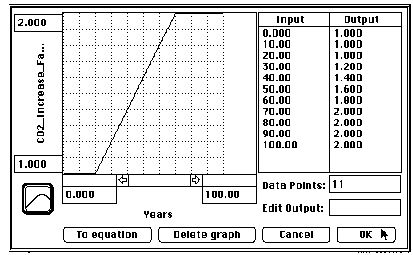
We allow 20 years for the system to re-equilibrate since our initial
conditions were not precise to many decimal places. The carbon dioxide
doubles linearly over 50 years. And, we allow another 30 years for the
system to reach a new steady state. This is a 100-year model run.
Run your model and display CO2 Increase Factor and Temperature; print
this result. Why is there a lag between the carbon dioxide change
and the temperature response? Write your answer to this question on the
printed graphic.
Now that your model is working we will use the sensitivity-study
capabilities of STELLA to compare the temperature outputs for 4 values
of H2O Factor, w, over the range 1.5 to 3.0. The
sensitivity dialog box is accessed from the Run Menu as Sensi Specs…;
here you will specify the number of comparative model runs and the
range of w. Print a copy of your system
diagram, your
equations, and your sensitivity runs
graphic results to submit as
part
of your assignment.
Comment.
Having completed the modeling exercise you see the necessity of
including an atmosphere in order to model the climate system. However,
a 1-layer atmosphere has serious limitations. For example, the maximum
greenhouse warming obtainable (a = 1) is 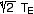 , which for the Earth would be
303 K or an increase of 48 K. We are at 33 K now, which is not far from
the limit. The greenhouse warming for Venus is ~500 K, an impossible
task for a 1-layer atmosphere.
, which for the Earth would be
303 K or an increase of 48 K. We are at 33 K now, which is not far from
the limit. The greenhouse warming for Venus is ~500 K, an impossible
task for a 1-layer atmosphere.
The more layers that are used to model the atmosphere the better the
approximation. Each layer can have a smaller absorption coefficient and
the perturbations are smaller. Every layer and the surface must
communicate by radiation with all of the others. A multi-layer model
has lots of plumbing!
Footnote.
A survey article, “Climate Modelers Struggle to
Understand Global
Warming,” (Physics Today, February, 1990, 17-19) provides
some
of the information that we need for this exercise.
Exercises 1 & 2 at the end of Section V of the System Behavior
and System Modeling develop much of the analytical descriptions of
the global energy balance model in the steady state, and the Instructor’s
Manual elaborates these solutions. The sensitivity equation for the
atmospheric absorption coefficient (p.37 in SB&SM and p. 18
&19 in IM) is
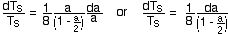
When integrated these equations give
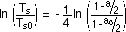
We can verify the consistency of the two approaches
to the problem by inserting the following values into this last
equation: Ts0
The differential form of the equation becomes: 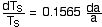
The absorption coefficient, a, represents all of the greenhouse
gases. We will separate it into two terms; a
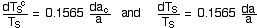
The ratio of the second to the first of these
equations yields
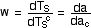
where w is the H2O factor in the range 1.5 to
3.0 obtained from the reading. We are somewhat closer to the answer but
not yet there. We have from above da = w * da
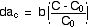
where b is a constant to be determined
empirically. Combining the results from the last four equations we find
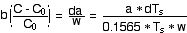
For a doubling of carbon dioxide (C - C0
thus b = 0.0171 to 0.02563. We will assume, therefore, that b
= 0.02, the CO2 Abs Calibration. Our empirical estimate is
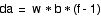
where f is the CO2 factor, i.e., C = f
* C0 . This last
equation is the one that we use in the model calculations.
#