Wednesday 20 th June 2007
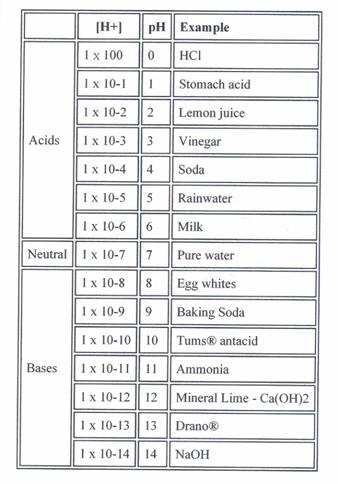
The pH of
Everyday Substances
Note: Safety goggles should be worn at all times during this lab activity.
Chemicals:
1. pH paper, pH indicator solution, pH probes linked to MicroLab
2. small beakers (100 mLs) from your drawer
3. Paper towels
4. Pipettes
5. substances to be tested:
1. water from Rice;
2. water from home;
3. lemon,
4. orange, and
5. grapefruit juices;
6. acetic acid (vinegar);
7. apple juice;
8. catsup (diluted);
9. ammonia (diluted 1 part ammonia to 10 parts water);
10. borax;
11. soft drinks – a selection, including diet coke;
12. milk of magnesia;
13. sodium bicarbonate (baking soda);
14. oven cleaner;
15. dish soap;
16. laundry detergent, shampoo,
17. soil in water,
18. rain water
(
19. pond water
20. also sea water
21. antacids
Procedure:


- We will demonstrate how to set-up pH meter and
how it works using pH 4, 7 and 10 to calibrate.
- Use indicator paper, indicator solution to
compare color and hence pH with the pH instrument.
- Label the beakers with the labels provided. Place approximately 2 mLs of each substance to be tested in the appropriate container. For example, place substance 1 in the beaker labeled 1. Continue with substances 2-x.
- The beaker labeled 7 is the control. The pH of this substance will be provided.
- Based on the list provided of the uses of substances 1-7, estimate the pH of each unknown and record the number in the data table in the estimated pH column.
- Test the pH of each substance, including 7, and record the actual pH number in the appropriate column in the data table.
- Determine if each substance has an acid, base or a neutral pH.
- Clean up and dispose of your unknown substances according to our instructions.
Data Table:
|
Substance |
Estimated pH Using indicator paper |
Actual pH Using pH meter |
Is it Acid, Base or Neutral? |
|
1. Water from Rice |
|
|
|
|
2. Deionised water |
|
|
|
|
3. Lemon |
|
|
|
|
4. Grapefruit Juice |
|
|
|
|
5. vinegar(acetic acid) |
|
|
|
|
6. Apple Juice |
|
|
|
|
7. Catsup (diluted) |
|
|
|
|
8. Ammonia |
|
|
|
|
9. Borax |
|
|
|
|
10. Coke |
|
|
|
|
11. Diet coke |
|
|
|
|
12. Sprite |
|
|
|
|
13. Milk of Magnesia |
|
|
|
|
14. sodium bicarbonate (baking soda) |
|
|
|
|
15. oven cleaner |
|
|
|
|
16. Dish soap |
|
|
|
|
17. Laundry detergent |
|
|
|
|
18. Shampoo |
|
|
|
|
19. Rain water ( |
|
|
|
|
20. Rain water (Rice) |
|
|
|
|
21. Orange juice (low acid) |
|
|
|
|
22. Orange juice |
|
|
|
Hypothesize about what will happen to the pH number if you mix an acid and a base together. In the hypothesis make a statement about how the pH will change.
My Hypothesis:
After the observations are complete, answer the following questions.
Conclusions:
1. How accurate were the estimated pH determinations in comparison to the actual pH determinations?
2. Which of the substances is the strongest acid? How did you make this determination?
3. Which of the substances is the strongest base? How did you make this determination?
4. If a substance has a pH of 3, which of the unknowns could be added to it to obtain a substance with a pH of approximately 7?
5. Based on your answer to #4, if an aquatic ecosystem has an acid pH, what could be done to neutralize it? Would this be a long term solution? Why or why not?
6. What effect do you think stomach acid has on some of the germs that enter our digestive tract?
pH Scale Assignment :
Order these items from low (acidic) to high
(basic) pH. Pure water has a neutral pH.
|
|
|
____ |
|
Lye |
|
|
|
____ |
|
Stomach Fluids |
|
|
|
____ |
|
Lemon Juice |
|
|
|
____ |
|
Vinegar |
|
|
|
____ |
|
Tomatoes |
|
|
|
____ |
|
Coffee |
|
|
|
____ |
|
Blood |
|
|
|
____ |
|
Pure Water |
|
|
|
____ |
|
Milk |
|
|
|
____ |
|
Rolaids, Tums |
|
|
|
____ |
|
Baking Soda |
|
|
|
____ |
|
Borax |
|
|
|
____ |
|
Household Ammonia |
|
|
|
____ |
|
Bleach |
Source: Borrowed from the Purdue lecture demonstration site:
http://chemed.chem.purdue.edu/demos/main_pages/17.4.html
Description:
Four beakers (or crystallizing dishes) are filled to the same level: two contain water while two contain acetate buffer. Indicators are added to each. Acid and base are added to the water-filled beakers and to the buffer-filled beakers. No color change can be observed in the buffer-filled beakers.
Materials:
- Four beakers or crystallizing dishes (for overhead projector)
- Acetate buffer:
- Fill 1000 mL volumetric with about 600 mL dH2O
- Add 172 mL glacial acetic acid
- Add 246 g sodium acetate
- Dilute to 1 L
- Four stirring rods
- 1 M NaOH
- 1 M HCl
- Beral pipets
Safety: Gloves and goggles
should be worn whenever working with acids or bases.
Procedure:
Set up the beakers as shown:
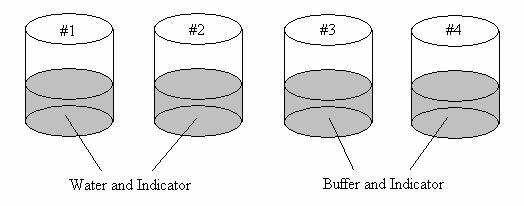
Add the 1 M HCl to beakers 1 and 3 using a pipet and stir with the stir rod provided. Add the 1 M NaOH to beakers 2 and 4 using a pipet and stir with the stir rod provided. The solutions in 3 and 4 should not change color, while the solutions in 1 and 2 should. Pour a little more HCl and NaOH from the bottle into beakers 3 and 4 respectively. These should not change the color until the buffer capacity has been reached.
Clean-Up:
The waste is safe to rinse down the drain with water.
Background:
The addition of strong acid or base to the buffer system will not affect the pH by a very large degree, since acid addition results in the production of a weaker acid and water, and base addition results in the production of a weaker base and water.
CH3COOH +
CH3COO- + H3O+ à CH3COOH + H2O (Addition of Acid)
Buffer systems in our bodies.
Buffers are important in everyday life because they
regulate the pH in our blood, keeping the pH between 7.35 and 7.45; if pH
values for our blood go outside this range, death can result. A buffer is composed of a weak acid and its
conjugate base (or a weak base and its conjugate acid). When a strong acid or base is added to a
buffer, one of the species will react to maintain the pH within a small range.