2024 Abstracts.
205. Hong, K., Rivera, D., Donoso, J., Shin, B., Jacobs, H., Byeong, J., Heck, K., Elias, W., Westerhoff, P. Han, Y., Muhich, C., Wong, M.S. "Nickel Enhances In Pd-Catalyzed Nitrate Reduction Activity and N2 Selectivity."ACS ES&T Engineering 2024DOI: https://doi.org/10.1021/acsestengg.4c00552

Palladium–indium (PdIn) is a well-established bimetallic composition for reductively degrading nitrate anions, one of the most ubiquitous contaminants in the groundwater. However, the scarcity and the variable price of these rare-earth and platinum group critical metals may hinder their use for water treatment. Nickel (Ni), a nonprecious metal in the same element group as Pd, could partially replace and lower Pd usage if the resulting trimetallic composition is sufficiently catalytically active. Herein, we report the synthesis and nitrate reduction catalysis of activated carbon-supported “In-on-Pd-on-Ni” catalysts (InPdNi/AC). While bimetallic InPd/AC (0.05 wt % In, 1.3 wt % Pd) was expectedly active, trimetallic InPdNi/AC containing the same In amount, much less Pd (0.1 wt %), and 1 wt % Ni was >17 more active (k cat ≈ 20 vs 349 L min -1 g surface metal -1). X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) calculations showed that Pd gained electron density from Ni, correlating to the increased nitrate reduction activity. Ammonium byproduct selectivity for InPdNi/AC (18% at 50% nitrate conversion) was lower compared to that of InPd/AC (48%), suggestive of the higher surface coverage of NO or its greater reactivity with NO2–, which led to more N2. Accounting for the catalyst precursor, manufacturing costs, and spent metal recovery, we calculated that Ni incorporation lowered the net catalyst cost significantly (from $1028/kg to $170/kg). The trimetallic composition lowered, by ∼26 times, the catalyst cost of a stirred tank reactor sized to the same treatment capacity as that for the bimetallic case. The results demonstrate that the partial replacement of the precious metal with an earth-abundant one leads to a higher efficiency and lower cost denitrification catalyst, via a material strategy that should be beneficial for other clean-water catalytic systems.
204. Juve, J.M.A., González, X.B., Bai, L., Xie, Z., Shang, Y., Saad, A., Gonzalez-Olmos, R., Wong, M.S., Ateia, M. and Wei, Z., 2024. “Size-selective trapping and photocatalytic degradation of PFOA in Fe-modified zeolite frameworks”.Applied Catalysis B: Environment and Energy 2024, 349, p.123885.DOI: https://doi.org/10.1016/j.apcatb.2024.123885

Removal and destruction of perfluorooctanoic acid (PFOA) are challenging due to its extreme persistence and dilute concentrations. This study investigated dual-function adsorptive-photocatalytic zeolite materials to selectively adsorb and degrade PFOA via tuning pore structures and doping transition metals. It is found that the pore opening is critical in the size-selective trapping of PFOA, while the iron doped zeolites present excellent adsorption of PFOA (>80 mg g-1) combining hydrophobic and electrostatic interactions. The formation of PFOA-iron complexes has reduced bond dissociation energy of C−F, calculated from density functional theory, for favorable stepwise defluorination (over 60% defluorination in 4 hours) by superoxide radicals and ligand-to-metal charge transfer. This mechanistic investigation extends the potential of the concentrate-and-degrade concept to remove PFOA selectively and effectively from contaminated water.
203. Cheng, Y., Deng, B., Scotland, P., Eddy, L., Hassan, A., Wang, B., Silva, K.J., Li, B., Wyss, K.M., Ucak-Astarlioglu, M.G. and Chen, J. “Electrothermal mineralization of per-and polyfluoroalkyl substances for soil remediation”Nature Communications 2024, 15(1), p.6117.DOI: https://doi.org/10.1038/s41467-024-49809-6

Per- and polyfluoroalkyl substances (PFAS) are persistent and bioaccumulative pollutants that can easily accumulate in soil, posing a threat to environment and human health. Current PFAS degradation processes often suffer from low efficiency, high energy and water consumption, or lack of generality. Here, we develop a rapid electrothermal mineralization (REM) process to remediate PFAS-contaminated soil. With environmentally compatible biochar as the conductive additive, the soil temperature increases to >1000 oC within seconds by current pulse input, converting PFAS to calcium fluoride with inherent calcium compounds in soil. This process is applicable for remediating various PFAS contaminants in soil, with high removal efficiencies ( >99%) and mineralization ratios ( >90%). While retaining soil particle size, composition, water infiltration rate, and cation exchange capacity, REM facilitates an increase of exchangeable nutrient supply and arthropod survival in soil, rendering it superior to the time-consuming calcination approach that severely degrades soil properties. REM is scaled up to remediate soil at two kilograms per batch and promising for large-scale, on-site soil remediation. Life-cycle assessment and techno-economic analysis demonstrate REM as an environmentally friendly and economic process, with a significant reduction of energy consumption, greenhouse gas emission, water consumption, and operation cost, when compared to existing soil remediation practices.
202. Li, C., Song, G., Lim, K.H., Hu, F., Sunarso, J., Yang, N., Wong, M.S., Liu, S. and Kawi, S., 2024. “Transcending catalytic limits for methane decomposition: multi-functional basalt fiber-supported catalysts with membrane synergy”.Advanced Composites and Hybrid Materials 2024, 7(3), p.99.DOI: https://doi.org/10.1007/s42114-024-00905-7

Catalytic methane (CH4) decomposition (CDM) offers a direct pathway for hydrogen (H2) gas production and valuable carbon nanotube (CNT) synthesis. However, the stability of this gas-to-solid reaction is hindered by limitations in CNT growth and reactor volume constraints. Departing beyond conventional nanopowder catalysts, we introduce basalt fiber-supported Ni/LTA catalysts that feature COx-free H2 generation and up to 3.7 times longer CDM reaction times, delivering an H2 production rate of 3.1 mol gNi-1h-1 over 22 h at 500 oC, surpassing Ni/LTA nanopowder counterparts. The basalt fiber catalysts exhibit uniform and robust CNT growth, along with sustained and stable H2 generation lasting up to three times longer relative to traditional CDM catalysts that deactivate within 10 h as reported in the literature. Integration of the flexible basalt fiber catalysts into an H2-permeable LTA-Pd membrane reactor further enhances the reaction time by 36% and CH4 conversion by 40%, achieving up to 45% CH4 conversion over 27 h, surpassing expected equilibrium conversion rates. The excellent catalytic stability of the 10 wt% Ni/LTA basalt fiber catalyst is additionally showcased through multiple reduction-800 °C CDM reaction-CO2 regeneration cycles. This transformative study propels the development of functional catalyst materials, revolutionizing thermocatalytic processes.
201. Yin, S.; López, Juan F.; Sandoval-Pauker, C.; Calvillo, J. J.; Glass, S.; Habib, A.; Lee, W.-Y.; Wong, M. S.; Pedro J.J. Alvarez; Villagrán, D. “Trap-n-Zap: Electrocatalytic Degradation of Perfluorooctanoic Acid (PFOA) with UiO-66 Modified Boron Nitride Electrodes at Environmentally Relevant Concentrations.”Applied Catalysis B: Environment and Energy2024, 124136–124136.DOI: https://doi.org/10.1016/j.apcatb.2024.124136

Poly- and per-fluoroalkyl substances (PFAS) are synthetic chemicals of increasing global concern due to their toxicity and environmental persistence. Hexagonal boron nitride (h-BN) is an electrochemically active semiconductor that generates a hydrophobic electron-hole with a high affinity for the fluorinated tail of perfluorooctanoic acid (PFOA), which enhances surface interactions in aqueous solutions. We demonstrate that h-BN electrocatalytically oxidizes PFOA to shorter chains. The degradation is enhanced by modifying the h-BN surface with an adsorbent, UiO-66 (UiO-66@BN), that facilitates proximal adsorption to catalytic sites (trap-and-zap). At the environmentally relevant concentration of 100 µg/L, electrochemical trap-and-zap removes 99.5% of PFOA with 70% defluorination in 3 h at a pH 4.5. Computational results show that PFOA requires an overpotential of 1.87 V vs. SCE, which is close to the experimental overpotential. The electrical energy per order (EE/O) of UiO-66@BN is 6.1 kWh/m3, indicating that it is a competitive material for PFOA electrochemical remediation.
200. Levi, J., Jung, B., Jacobs, H. P., Luo, Y., Lee, C. S., Hong, K., Long, M., Donoso, J., Garcia-Segura, S., Wong, M., Rittmann, B., & Westerhoff, P. “Optimized bimetallic ratios for durable membrane catalyst-film reactors in treating nitrate-polluted water”Science of The Total Environment(2024): 173711.DOI: https://doi.org/10.1016/j.scitotenv.2024.173711

Nitrate contamination of surface and ground water is a significant global challenge. Most current treatment technologies separate nitrate from water, resulting in concentrated waste streams that need to be managed. Membrane Catalyst-film Reactors (MCfR), which utilize in-situ produced nanocatalysts attached to hydrogen-gas-permeable hollow-fiber membranes, offer a promising alternative for denitrification without generating a concentrated wastestream. In hydrogen-based MCfRs, bimetallic nano-scale catalysts reduce nitrate to nitrite and then further to di-nitrogen or ammonium. This study first investigated how different molar ratios of indium-to-palladium (In:Pd) catalytic films influenced denitrification rates in batch-mode MCfRs. We evaluated eleven In-Pd bimetallic catalyst films, with In:Pd molar ratios from 0.0029 to 0.28. Nitrate-removal exhibited a volcano-shaped dependence on In content, with the highest nitrate removal (0.19 mgNO3--N-min-1L-1) occurring at 0.045 mol In/mol Pd. Using MCfRs with the optimal In:Pd loading, we treated nitrate-spiked tap water in continuous-flow for >60 days. Nitrate removal and reduction occurred in three stages: substantial denitrification in the first stage, a decline in denitrification efficiency in the second stage, and stabilized denitrification in the third stage. Factors contributing to the slowdown of denitrification were: loss of Pd and In catalysts from the membrane surface and elevated pH due to hydroxide ion production. Sustained nitrate removal will require that these factors be mitigated.
199. Kim, D., Byeong, J., Guo, H., Gao, G., Pennington, C., Wong, M., Getachew, B., & Han, Y. “Precise Fabrication and Manipulation of Individual Polymer Nanofibers”Nano Letters2024, 24, 20, 6038–6042DOI: https://doi.org/10.1021/acs.nanolett.4c00799

Polymer nanofibers hold promise in a wide range of applications owing to their diverse properties, flexibility, and cost effectiveness. In this study, we introduce a polymer nanofiber drawing process in a scanning electron microscope and focused ion beam (SEM/FIB) instrument with in situ observation. We employed a nanometer-sharp tungsten needle and prepolymer microcapsules to enable nanofiber drawing in a vacuum environment. This method produces individual polymer nanofibers with diameters as small as ∼500 nm and lengths extending to millimeters, yielding nanofibers with an aspect ratio of 2000:1. The attachment to the tungsten manipulator ensures accurate transfer of the polymer nanofiber to diverse substrate types as well as fabrication of assembled structures. Our findings provide valuable insights into ultrafine polymer fiber drawing, paving the way for high-precision manipulation and assembly of polymer nanofibers.
198. Wang, B., Chen, Y., Samba, J., Heck, K., Huang, X., Lee, J.,Metz, J., Bhati, M., Fortner, J., Lin, Q., Westerhoff, P., Alvarez, P., Senftle, T., & Wong, M. S. "Surface hydrophobicity of boron nitride promotes PFOA photocatalytic degradation"Chemical Engineering Journal483 (2024): 149134.DOI: https://doi.org/10.1016/j.cej.2024.149134
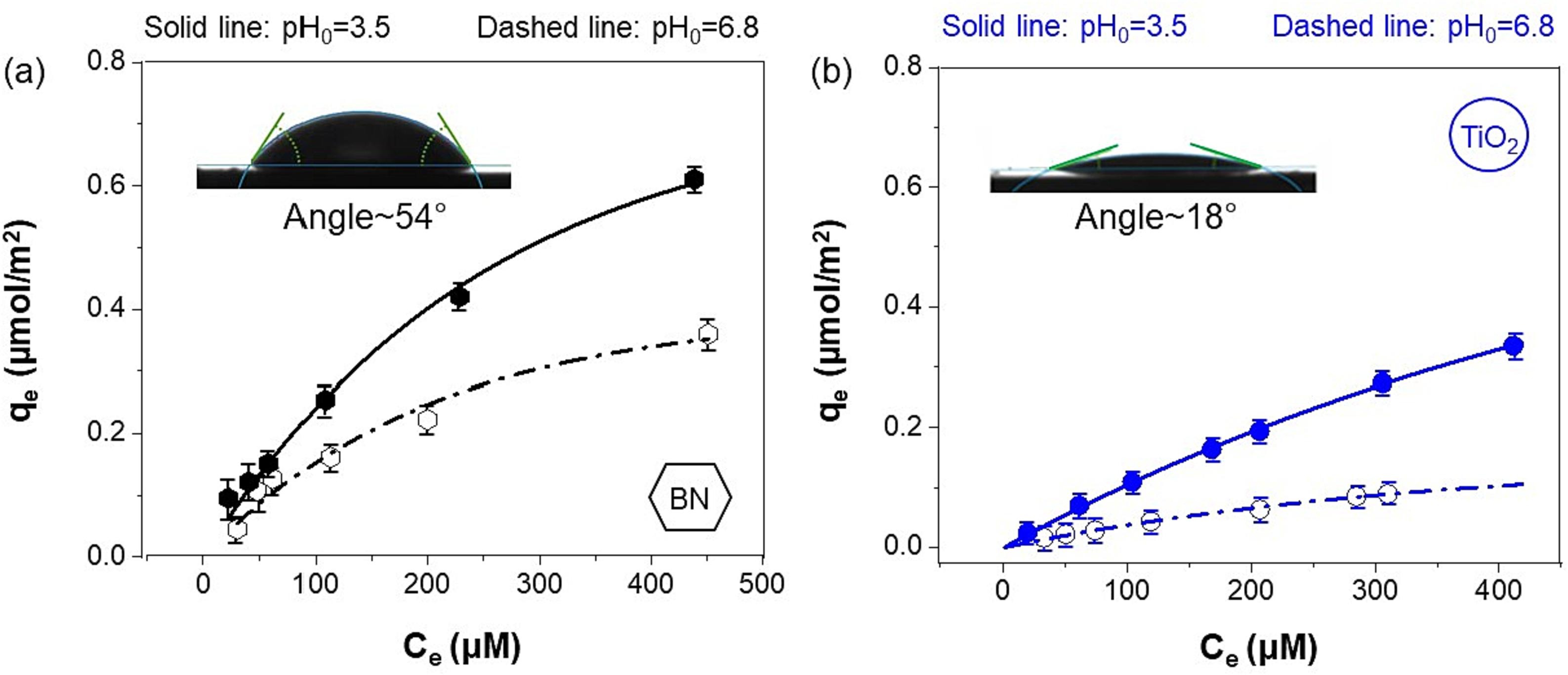
Boron nitride (BN) photodegrades perfluorooctanoic acid (PFOA) in water under 254-nm light illumination more rapidly than TiO2, which is hypothesized due to its greater surface hydrophobicity. We investigated the role of hydrophobicity on PFOA photocatalysis by comparing BN with anatase TiO2 under reaction conditions, for which the exposed surface areas were the same. BN exhibited ∼ 3.5 × faster PFOA degradation rate compared to TiO2 under acidic pH conditions. PFOA adsorption experiments showed that BN had ∼ 2 × higher PFOA surface coverage, consistent with its higher surface hydrophobicity, as corroborated by contact angle measurements. Both materials were comparatively less photocatalytically active at neutral pH, but BN still exhibited ∼ 2.7 × faster PFOA degradation rate, due to less electrostatic attraction between the PFOA headgroup and the catalyst surface. Langmuir-Hinshelwood rate law analysis suggests BN and TiO2 have comparable photogenerated hole surface concentrations, and density functional theory calculations show that the holes for both photocatalysts can react with surface hydroxyls and with adsorbed PFOA. However, BN has comparatively less surface hydroxyl groups and more adsorbed PFOA, which favors hole reaction with the latter, resulting in a higher PFOA degradation rate. These insights into the role of surface hydrophobicity serve as rationally-guided design principles for improved heterogeneous photocatalysis of persistent surfactants, including the broad suite of per- or poly-fluoroalkyl substances.
196. Long, M., Zhou, Ch., Elias, W., Jacobs, H., Heck, K., Wong, M., Rittmann, B. “Auto-Assembled Pd–Rh Nanoalloys Catalyzed Faster and Deeper Hydrodefluorination of Perfluorooctanoic Acid (PFOA) in Environmental Conditions”ACS ES&T Engineering2024DOI: https://doi.org/10.1021/acsestengg.3c00548
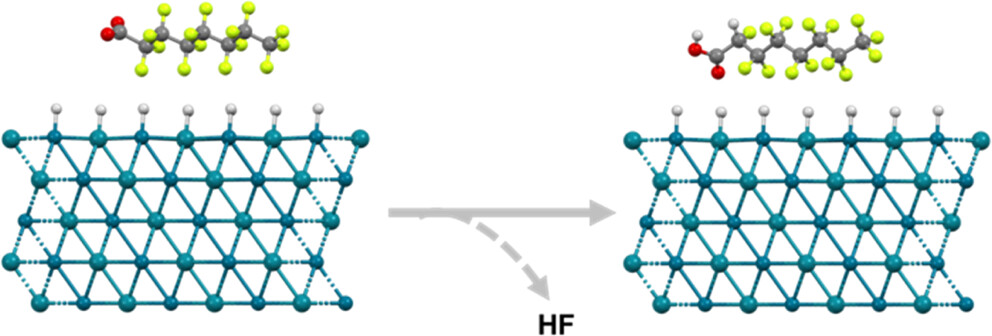
Perfluoroalkyl substances (PFASs) are drawing attention because of their widespread contamination in waters and their risks to human and ecosystem health at low concentrations. We evaluated auto-assembled palladium (Pd) plus rhodium (Rh) nanoalloys for H2-induced catalytic hydrodefluorination for one of the most prominent PFASs, perfluorooctanoic acid (PFOA), at neutral pH and ambient temperature. Nanoalloys of Pd and Rh displayed enhanced hydrodefluorination capacity compared to Pd and Rh mononanoparticles. Compared to Rh, Pd–Rh retained the similar specific hydrodefluorination ratio but yielded a 5-fold higher hydrodefluorination efficiency due to the stronger adsorption capacity from Pd. Compared to Pd, Pd–Rh showed a slower PFOA removal rate, but its hydrodefluorination capacity was enhanced 12-fold due to the presence of Rh in the alloy. Correspondingly, the completely defluorinated product, octanoic acid, became the dominant product of hydrodefluorination with the Pd–Rh alloy. In continuous-flow tests at pH 7, the bimetallic Pd–Rh catalysts exhibited better and longer-lasting PFOA removal and hydrodefluorination compared to mono-Pd and -Rh catalysts. Atom-scale modeling using density functional theory (DFT) explained the synergistic effect of nanoalloys in adsorbing and C–F dissociation of PFOA at neutral pH. The experimental results and thermodynamic modeling support that Pd–Rh nanoalloys have promise for detoxifying PFOA in environmentally relevant conditions.
195. Chung, Y., Jang, D., Kim, H., Oh, G., Tak, H., Son, C., Jeon, M., Chae, S., Hong, Y., Wong, M., Kang, S. “Occurrence and Treatment of DEHP in Water Treatment Plants: Case study in South Korea”ACS ES&T Engineering2024DOI: https://doi.org/10.1021/acsestengg.3c00396
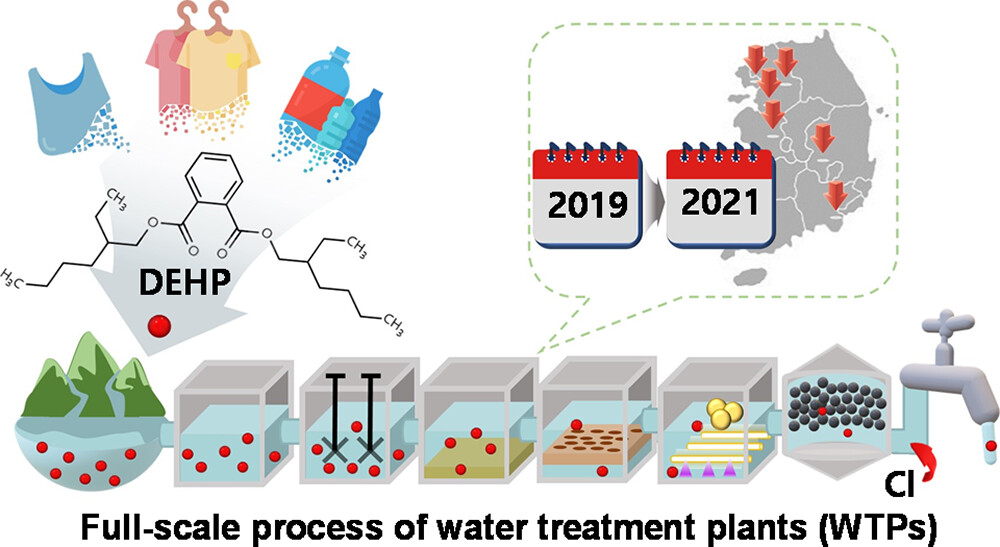
Currently, a toxic plasticizer, di(2-ethylhexyl) phthalate (DEHP), is recognized as a ubiquitous pollutant in aquatic environments due to the massive utilization of plastics. However, there is still a limited understanding of the occurrence and treatment of DEHP during the potable water utilization due to a lack of long-term field data in the full-scale process of water treatment plants (WTPs). In this study, we investigated the occurrence and removal of DEHP in six conventional WTPs located at various river basins in South Korea from June 2019 to December 2021. During the observation periods, DEHP was found in all samples at concentrations ranging from 0.3 to 8.2 μg/L. Among the unit processes, the removal of DEHP was positively correlated with dissolved organic carbon removal, majorly in sand filtration and the GAC process. The advanced oxidation process exhibited low selectivity for DEHP removal efficiencies in the presence of natural organic matter due to its inhibition effect. The potential risks of DEHP on human health still exist in the surface water of South Korea for drinking water supplies, and thus, DEHP needs to be carefully monitored throughout the entire water treatment process.
194. Long, M., Chen, Y., Senftle, T., Elias, W., Heck, K., Zhou, C., Wong, M., Rittmann, B. “Method of H2 Transfer Is Vital for Catalytic Hydrodefluorination of Perfluorooctanoic Acid (PFOA)”Environmental Science & Technology2024, 58, 2, 1390–1398DOI: https://doi.org/10.1021/acs.est.3c07650
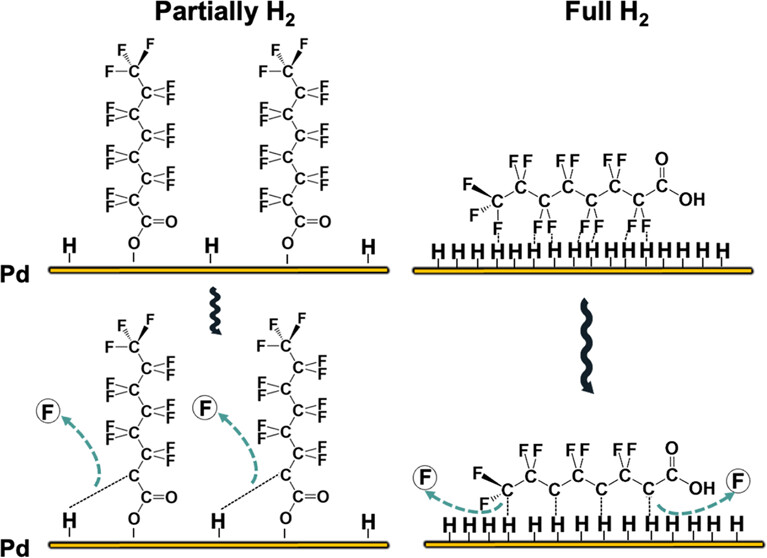
The efficient transfer of H2 plays a critical role in catalytic hydrogenation, particularly for the removal of recalcitrant contaminants from water. One of the most persistent contaminants, perfluorooctanoic acid (PFOA), was used to investigate how the method of H2 transfer affected the catalytic hydrodefluorination ability of elemental palladium nanoparticles (PdoNPs). PdoNPs were synthesized through an in situ autocatalytic reduction of Pd2+ driven by H2 from the membrane. The Pdo nanoparticles were directly deposited onto the membrane fibers to form the catalyst film. Direct delivery of H2 to PdoNPs through the walls of nonporous gas transfer membranes enhanced the hydrodefluorination of PFOA, compared to delivering H2 through the headspace. A higher H2 lumen pressure (20 vs 5 psig) also significantly increased the defluorination rate, although 5 psig H2 flux was sufficient for full reductive defluorination of PFOA. Calculations made using density functional theory (DFT) suggest that subsurface hydrogen delivered directly from the membrane increases and accelerates hydrodefluorination by creating a higher coverage of reactive hydrogen species on the PdoNP catalyst compared to H2 delivery through the headspace. This study documents the crucial role of the H2 transfer method in the catalytic hydrogenation of PFOA and provides mechanistic insights into how membrane delivery accelerates hydrodefluorination.
192. Ersan, M., Wang, B., Wong, M., Westerhoff, P.” Advanced oxidation processes may transform unknown PFAS in groundwater into known products”Chemosphere 349 (2024): 140865 DOI: https://doi.org/10.1016/j.chemosphere.2023.140865
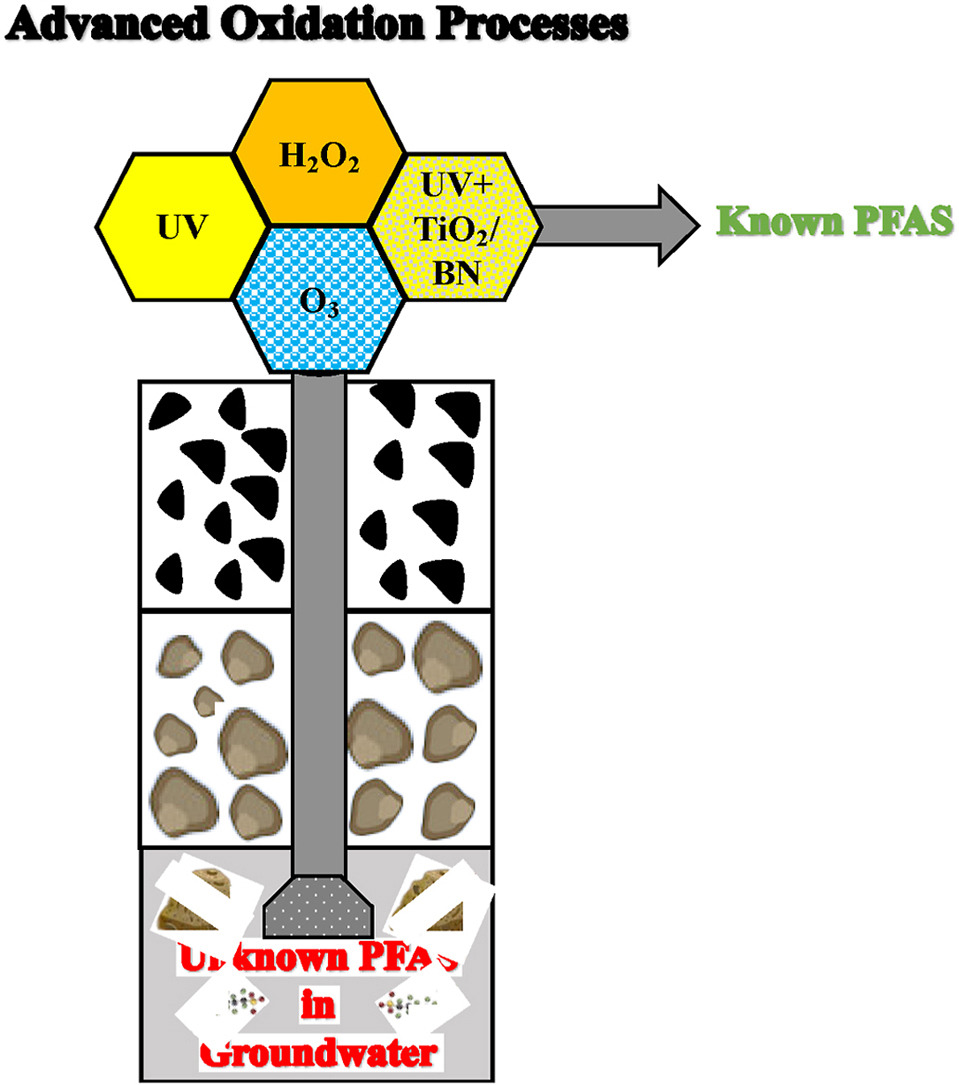
Per- and polyfluoroalkyl substances (PFAS) are a group of fluorinated organic contaminants classified as persistent in the aquatic environment. Early studies using targeted analysis approaches to evaluate the degradation of PFAS by advanced oxidation processes (AOP) in real water matrices may have been misinterpreted due to the presence of undetected or unknown PFAS in these matrices. The aims of the present study were to (1) screen selected commercially available AOPs (UV, UV + H2O2, O3/H2O2) and UV photocatalysis in a pilot system using commercially used and novel photocatalysts (TiO2, boron nitride [BN]) for removing PFAS contaminants and (2) evaluate their role on the conversion of non-detected/unknown to known PFAS compounds in real groundwater used as drinking water supplies. Results indicated that, while AOPs have the potential to achieve removal of the EPA method 533 target PFAS compounds (PFDA [100%], PFNA [100%], PFOA [85–94%], PFOS [25–100%], PFHxS [3–100%], PFPeS [100%], PFBS [100%]), AOPs transformed non-detected/unknown longer-chain PFAS compounds to detectable shorter-chain ones under very high-dose AOP operating conditions, leading to an increase in ∑PFAS concentration ranging from 95% to 340%. As emerging PFAS treatment processes transition from lab-scale investigations of target PFAS to pilot testing of real water matrices, studies will need to consider impact of the presence of non-target long-chain PFAS to transform into targeted PFAS compounds. A promising approach to address the potential risks and unforeseen consequences could involve an increased reliance on adsorbable organic fluorine (AOF) analysis before and after advanced oxidation process (AOP) treatment.
191. Jacobs, H., Elias, W., Heck, K., Dean, D., Dodson, J., Zhang, W., Hong, K., Arredondo, J., Breckner, C., Chen, L., Mueller, S., Alexander, S., Miller, J., Wong, M.ACS Catalysis 2024, 14, 1, 211–226 DOI: https://doi.org/10.1021/acscatal.3c04335
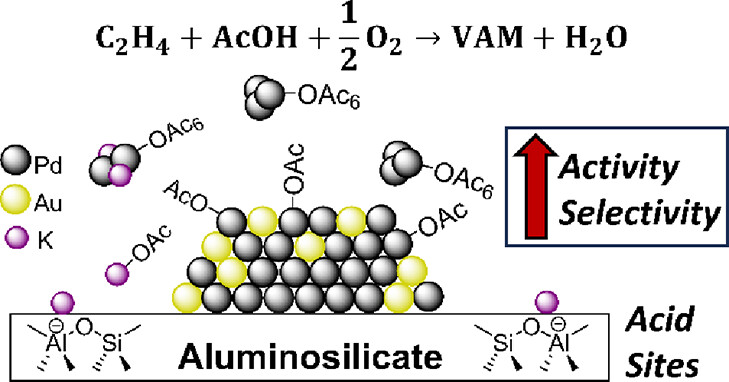
PdAu catalysts supported on both pure silica and aluminosilicates and promoted with KOAc are the most active and selective compositions for the industrial-scale acetoxylation of ethylene with acetic acid (AcOH) to form vinyl acetate. However, the effect of surface acidity on this reaction has not been systematically studied. In this work, we prepared PdAu (8:4 wt % Pd/Au) and KOAc-promoted PdAu (8:4:3 wt % Pd/Au/K) model catalysts using pure silica and aluminosilicate (10 wt % Al2O3, “Siral90”) as supports. XRD showed that the four fresh catalysts (PdAu/SiO2, PdAu/KOAc/SiO2, PdAu/Siral90, and PdAu/KOAc/Siral90) contained two distinct phase compositions: a nearly pure Au phase and a Pd-rich alloy phase. XRD grain sizes were noticeably larger for the Pd-rich alloy phase on the aluminosilicate materials compared with the silica-supported ones. The overall Pd surface atom content was lower and more dispersed on the aluminosilicate-supported catalysts based on XPS, XAS, and CO chemisorption analyses. In the presence of KOAc, the overall Pd surface atom content decreased and became more dispersed on both the silica- and aluminosilicate-supported catalysts. DRIFTS of fresh PdAu/KOAc/Siral90 showed the formation of Pd3(OAc)6 and surface-adsorbed acetate species, which were absent on PdAu/KOAc/SiO2. Operando DRIFTS-GC of PdAu/KOAc/Siral90 under reactant gas flow showed higher activity and selectivity to vinyl acetate compared to the silica-only case, which correlated to increased amounts of Pd3(OAc)6 and surface-adsorbed acetate. In situ DRIFTS revealed the presence of K2Pd2(OAc)6 on PdAu/KOAc/Siral90 during exposure to inert, oxygen, or AcOH gas conditions. In situ XRD showed the formation of a third crystal phase (∼Pd50Au50) under different gas conditions. We find that acid sites alter the catalyst nanostructure and promote the formation of Pd-acetate surface species, leading to greater activity and selectivity and illustrating how surface acidity contributes to PdAu-catalyzed vinyl acetate production.