2023 Abstracts.
188. Lee, J., Kim, C., Liu, C., Wong, M. S., Cápiro, N. L., Pennell, K. D., & Fortner, J. D. “Ultra-high capacity, multifunctional nanoscale sorbents for PFOA and PFOS treatment”.npj Clean Water , 6(1), 62 (2023).DOI: https://doi.org/10.1038/s41545-023-00263-9
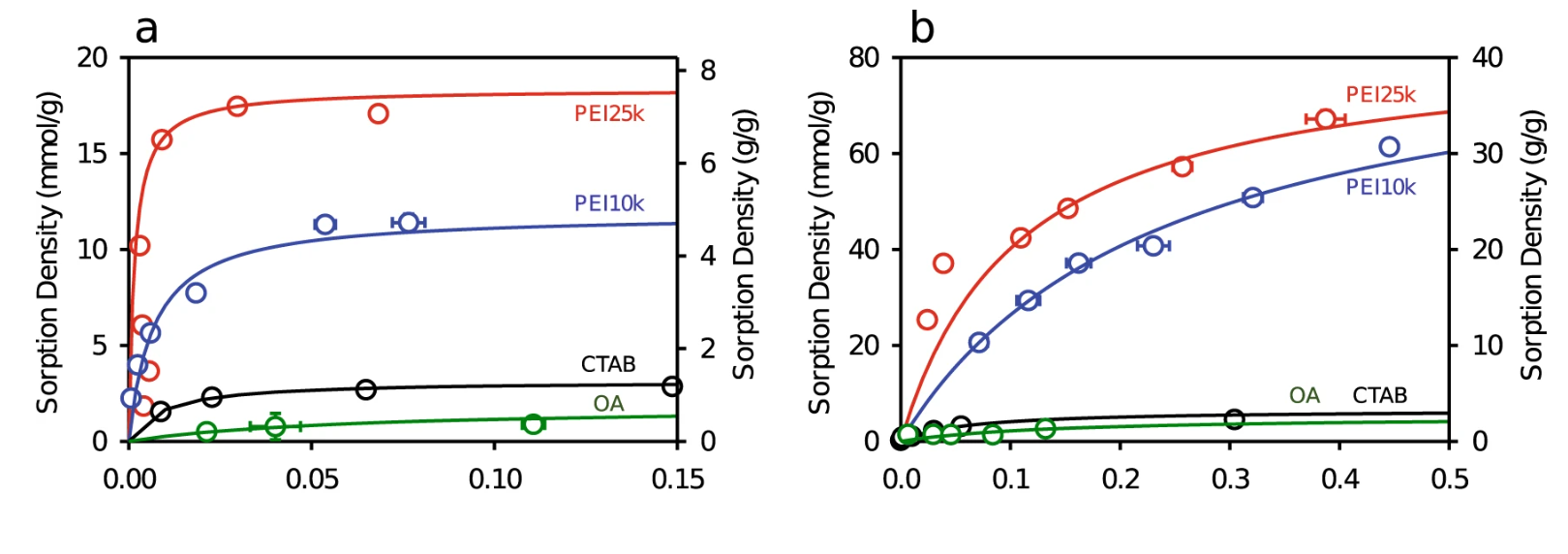
Here, we describe surface functionalized, superparamagnetic iron oxide nanocrystals (IONCs) for ultra-high PFAS sorption and precise, low energy (magnetic) separation, considering perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). As a function of surface coating, sorption capacities described are considerably higher than previous studies using activated carbon, polymers, and unmodified metal/metal oxides, among others. In particular, positively charged polyethyleneimine (PEI) coated IONCs demonstrate extreme sorption capacities for both PFOA and PFOS due to electrostatic and hydrophobic interactions, along with high polymer grafting densities, while remaining stable in water, thus maintaining available surface area. Further, through a newly developed method using a quart crystal microbalance with dissipation (QCM-D), we present real-time, interfacial observations (e.g., sorption kinetics). Through this method, we explore underpinning mechanism(s) for differential PFAS (PFOA vs PFOS) sorption behavior(s), demonstrating that PFAS functional head group strongly influence molecular orientation on/at the sorbent interface. The effects of water chemistry, including pH, ionic composition of water, and natural organic matter on sorption behavior are also evaluated and along with material (treatment) demonstration via bench-scale column studies.
187. Juve, J. M. A., Reece, J. A. D., Wong, M. S., Wei, Z., & Ateia, M.. “Photocatalysts for Chemical-Free PFOA Degradation–What we know and where we go from here?”.Journal of Hazardous Materials 132651 (2023). DOI: https://doi.org/10.1016/j.jhazmat.2023.132651
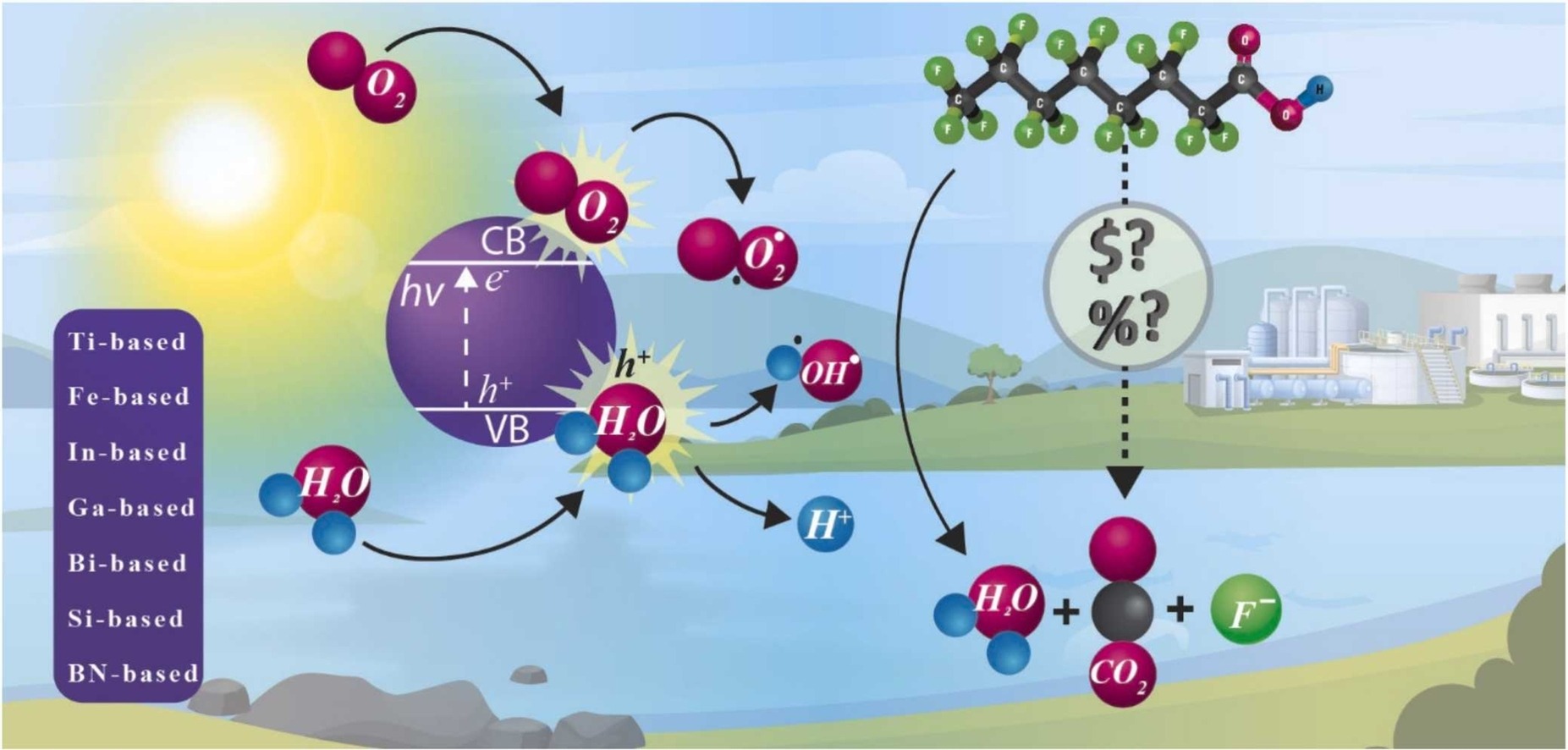
Perfluorooctanoic acid (PFOA) is a toxic and recalcitrant perfluoroalkyl substance commonly detected in the environment. Its low concentration challenges the development of effective degradation techniques, which demands intensive chemical and energy consumption. The recent stringent health advisories and the upgrowth and advances in photocatalytic technologies claim the need to evaluate and compare the state-of-the-art. Among these systems, chemical-free photocatalysis emerges as a cost-effective and sustainable solution for PFOA degradation and potentially other perfluorinated carboxylic acids. This review (I) classifies the state-of-the-art of chemical-free photocatalysts for PFOA degradation in families of materials (Ti, Fe, In, Ga, Bi, Si, and BN), (II) describes the evolution of catalysts, identifies and discusses the strategies to enhance their performance, (III) proposes a simplified cost evaluation tool for simple techno-economical analysis of the materials; (IV) compares the features of the catalysts expanding the classic degradation focus to other essential parameters, and (V) identifies current research gaps and future research opportunities to enhance the photocatalyst performance. We aim that this critical review will assist researchers and practitioners to develop rational photocatalyst designs and identify research gaps for green and effective PFAS degradation.
186. Elias, W., Clark, C., Heck, K., Arredondo, J., Wang, B., Toro, A., Kürti, L., Wong, M., “Niobium Oxide Photocatalytically Oxidizes Ammonia in Water at Ambient Conditions” J. Braz. Chem. Soc., Vol. 00, No. 00, e-20230163, 1-9 (2023).DOI: https://dx.doi.org/10.21577/0103-5053.20230163
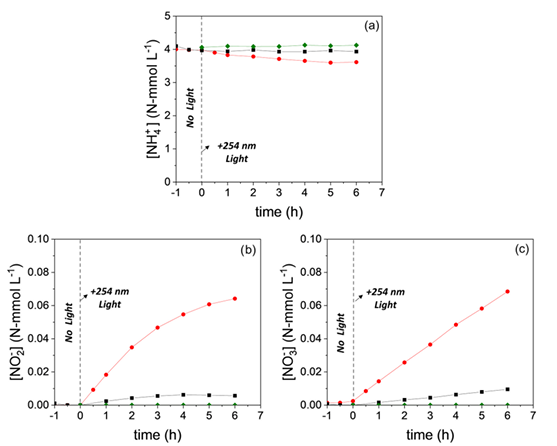
Ammonia contamination in water is a significant environmental issue since it is toxic and leads to eutrophication. Photocatalysis has been investigated as a strategy for ammonia degradation but can potentially form toxic nitrite (NO2- ) and nitrate (NO3- ) byproducts. This work reports on the ability of niobium oxide (Nb2O5 ) to photocatalytically oxidize aqueous-phase ammonia (NH3 ). Whereas as-synthesized Nb2O5 showed little catalytic activity (<1% NH3 conversion after 6 h of UV-C irradiation, at room temperature and atmospheric pressure, and under O2 headspace), Nb2O5 treated in basic solution (OH-Nb2O5 ) was able to photocatalytically degrade NH3 at ca. 9% conversion after six hours, with ca. 70% selectivity to the desired N2 , with a first-order rate constant of ca. 12 times higher than the as synthesize catalyst (1.6 × 10-3 min-1 vs. 2.0 × 10-2 min-1). Raman spectroscopic analysis indicated the presence of terminal Nb=O species after base treatment of Nb2O5, implicating them as catalytically active sites. These results underscore how a simple structural modification can significantly affect photocatalytic activity for aqueous ammonia oxidation.
185. Fehr, Austin MK, Todd G. Deutsch, Francesca M. Toma, Michael S. Wong, and Aditya D. Mohite. "Technoeconomic Model and Pathway to< $2/kg Green Hydrogen Using Integrated Halide Perovskite Photoelectrochemical Cells."ACS Energy Letters 8 (2023): 4976-4983. DOI: https://doi.org/10.1021/acsenergylett.3c01865
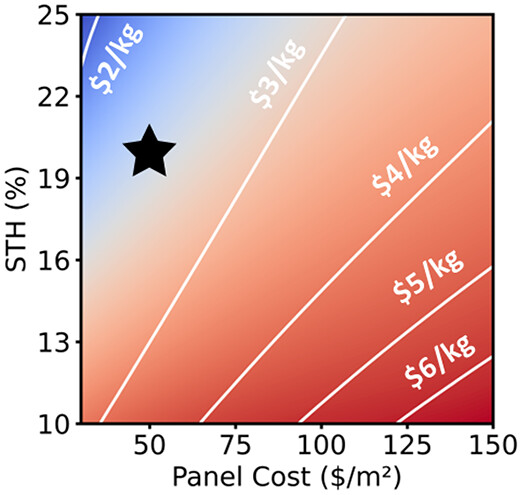
The cost of gray hydrogen produced via fossil fuel-based steam-methane reforming has led the U.S. Department of Energy to specify <$2/kg H2 as a target for commercially competitive green hydrogen generation methods. Integrated photoelectrochemical cells have been proposed as a solar-to-hydrogen conversion technology. Here, we describe a technoeconomically feasible pathway to reaching <$2/kg green H2 using integrated photoelectrochemical cells with halide perovskite photoabsorbers, low-cost conductive barriers, and low precious metal-content catalysts in an aqueous, membrane-separated cell. A base-case solar-to-hydrogen conversion efficiency of 20%, stable lifetime of 10 years, and a combined electrocatalyst-plus-panel cost of $50/m2 enabled a levelized cost of hydrogen of $2.43/kg, which dropped below $2/kg with improved performance metrics including material cost, improvements in process design, or subsidies. We relate these metrics to lab-scale reports to recommend best research practices for scientists and funding agencies working at this intersection of photovoltaics, electrocatalysis, and surface science.
184. Juve, J. M. A., Wang, B., Wong, M. S., Ateia, M., Wei, Z.”Complete defluorination of per-and polyfluoroalkyl substances—dream or reality?”.Current Opinion in Chemical Engineering 41, 100943 (2023) DOI: doi.org/10.1016/j.coche.2023.100943
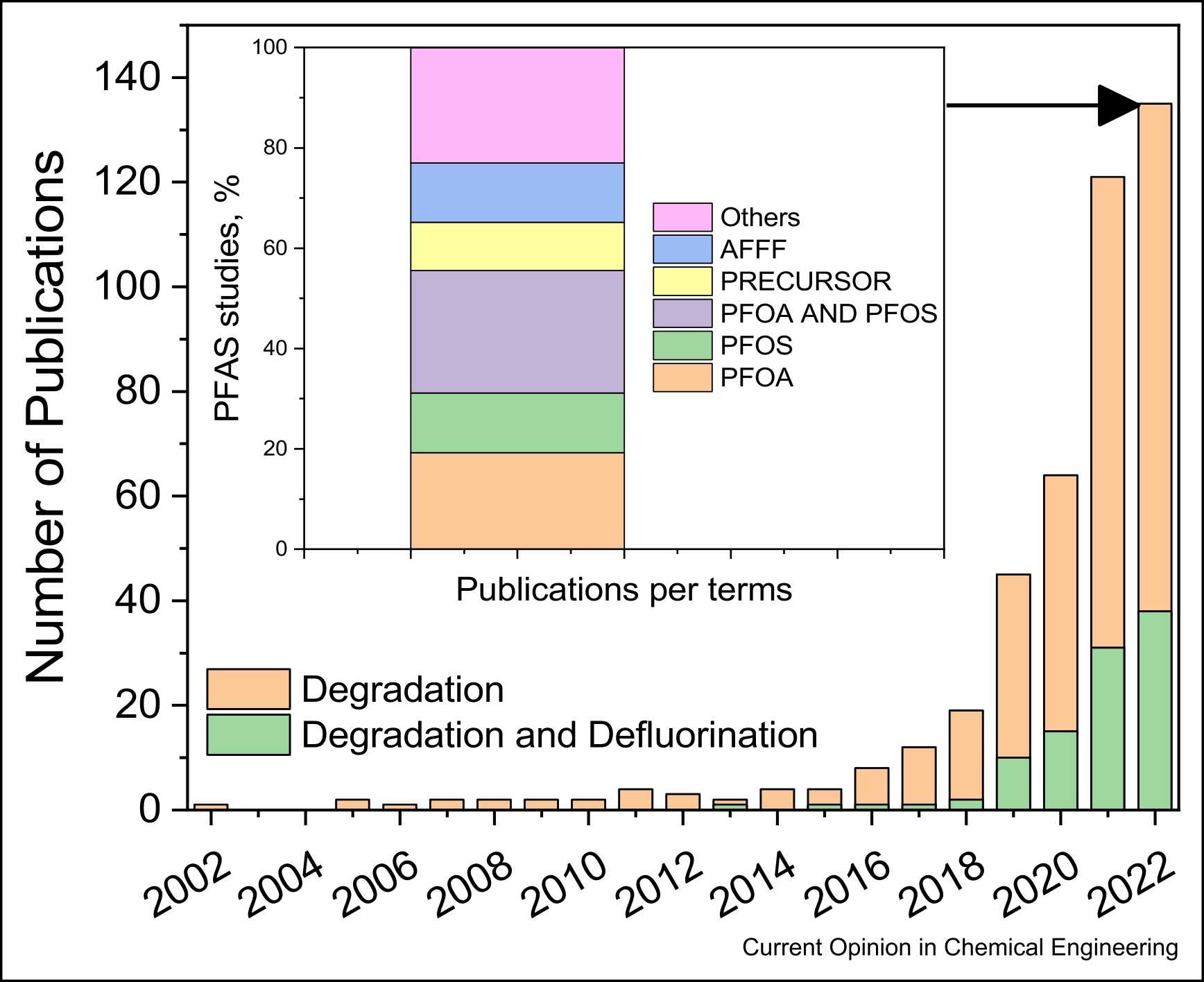
The consensus of removing per- and polyfluoroalkyl substances (PFAS) from the environment is widely recognized and enlightened by the near-zero standards released from the U.S. Environmental Protection Agency in 2023. The only way to achieve the goal of zero fluoro-pollution is to fully defluorinate or mineralize PFAS, but current technologies only partially defluorinate a limited number of PFAS, which can lead to the creation of potentially more toxic short-chain intermediates. Therefore, we discuss herein the need to broaden the scope of tested PFAS, summarize the state-of-the-art degradation technologies, and provide perspectives to achieve complete defluorination. Besides fundamental knowledge gaps in defluorination reactions, technological gaps in the aspects of water matrix effects, pilot tests, and cost analysis also limit the application and comparison of different treatment technologies. This work would shed light on further research to find solutions in the complete defluorination of PFAS.
183. Qanbarzadeh, M., DiGiacomo, L., Bouteh, E., Alhamdan, E. Z., Mason, M. M., Wang, B., Wong, M. S, Cates, E. L. “An Ultraviolet/Boron Nitride Photocatalytic Process Efficiently Degrades Poly-/Perfluoroalkyl Substances in Complex Water Matrices”Environmental Science & Technology Letters Article ASAP (2023) DOI: doi.org/10.1021/acs.estlett.3c00363
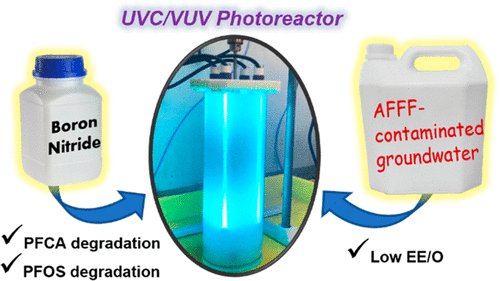
Hexagonal boron nitride (hBN) is among several semiconductor photocatalysts shown to degrade poly-/perfluoroalkyl substances (PFAS) in aqueous suspension when excited by ultraviolet-C radiation (UVC; 254 nm); however, only degradation of perfluorooctanoic acid (PFOA) at ppm-range concentrations has been examined in previous reports, showing relatively low efficiency. Herein, photocatalytic treatment of PFOA and other PFAS by hBN was found to be much more efficient than other catalysts when applied to environmentally relevant concentrations in the ppb range. The unexpected inverse concentration dependence is likely related to the contribution of hydrophobic interactions to PFAS adsorption on hBN, as are the observed wider operational pH and salinity ranges. When excited with vacuum ultraviolet-emitting lamps (185/254 nm), hBN was also able to degrade perfluorooctanesulfonate (PFOS) through an unconventional mechanism. Treatment of groundwater contaminated by aqueous film-forming foams was demonstrated in a 5L hBN+UVC/VUV photoreactor, achieving >99% degradation of PFOA in 15 min and 65% degradation of PFOS in 1 h. Electrical energies per order of destruction for PFOA and PFOS therein were 2.7 and ~50 kWh•m-3, respectively. The strong performance under a realistic water matrix and operational conditions suggests that hBN photocatalysis warrants further research and consideration as a treatment tool for PFAS contamination.
182. A. Fehr, A. Agrawal, F. Mandani, C. Conrad, Q. Jiang, S. Park, O. Alley,. B. Li, S. Sidhik, I. Metcalf, C. Botello, J. Young, J. Even, J. Blancon, T. Deutsch, K. Zhu, S. Albrecht, F. Toma, M. Wong, A. Mohite. "Integrated halide perovskite photoelectrochemical cells with solar-driven water-splitting efficiency of 20.8%"Nature Communications 14, 3797 (2023) DOI: 10.1038/s41467-023-39290-y
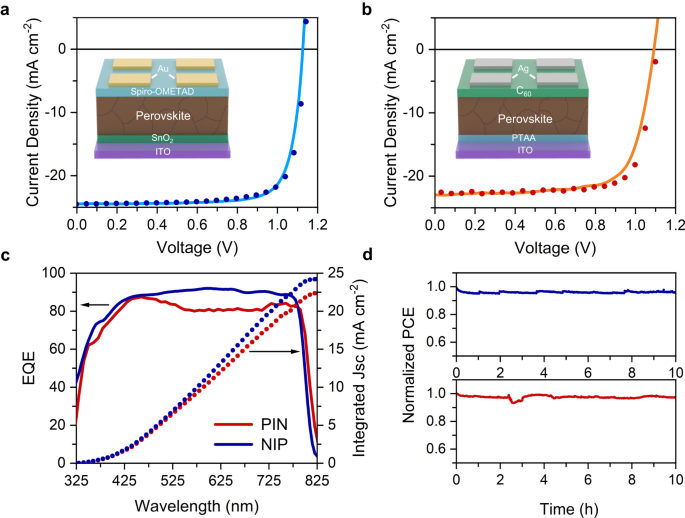
Achieving high solar-to-hydrogen (STH) efficiency concomitant with long-term durability using low-cost, scalable photo-absorbers is a long-standing challenge. Here we report the design and fabrication of a conductive adhesive-barrier (CAB) that translates >99% of photoelectric power to chemical reactions. The CAB enables halide perovskite-based photoelectrochemical cells with two different architectures that exhibit record STH efficiencies. The first, a co-planar photocathode-photoanode architecture, achieved an STH efficiency of 13.4% and 16.3 h to t60, solely limited by the hygroscopic hole transport layer in the n-i-p device. The second was formed using a monolithic stacked silicon-perovskite tandem, with a peak STH efficiency of 20.8% and 102h of continuous operation before t60 under AM 1.5G illumination. These advances will lead to efficient, durable, and low-cost solar-driven water-splitting technology with multifunctional barriers.
181. X. Wu, M. Nazemi, S. Gupta, A. Chismar, K. Hong, H.P. Jacobs, W. Zhang,KaliRigby, T. Hedtke, Q. Wang, E. Stavitski, M.S.Wong, C. Muhich,and J.H. Kim. "Contrasting Capability of Single Atom Palladium for Thermocatalytic versus Electrocatalytic Nitrate Reduction Reaction"ACS Catalysis 3, 6804–6812 (2023) DOI: 10.1021/acscatal.3c01285
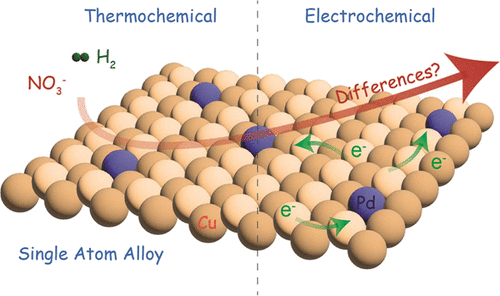
The occurrence of high concentrations of nitrate in various water resources is a significant environmental and human health threat, demanding effective removal technologies. Single atom alloys (SAAs) have emerged as a promising bimetallic material architecture in various thermocatalytic and electrocatalytic schemes including nitrate reduction reaction (NRR). This study suggests that there exists a stark contrast between thermocatalytic (T-NRR) and electrocatalytic (E-NRR) pathways that resulted in dramatic differences in SAA performances. Among Pd/Cu nanoalloys with varying Pd–Cu ratios from 1:100 to 100:1, Pd/Cu(1:100) SAA exhibited the greatest activity (TOFPd = 2 min–1) and highest N2 selectivity (94%) for E-NRR, while the same SAA performed poorly for T-NRR as compared to other nanoalloy counterparts. DFT calculations demonstrate that the improved performance and N2 selectivity of Pd/Cu(1:100) in E-NRR compared to T-NRR originate from the higher stability of NO3* in electrocatalysis and a lower N2 formation barrier than NH due to localized pH effects and the ability to extract protons from water. This study establishes the performance and mechanistic differences of SAA and nanoalloys for T-NRR versus E-NRR.
180. J. Levi, S. Guo, S. Kavadiya, Y. Luo, C. Lee, H.P. Jacobs, Z. Holman, M.S. Wong, S. Garcia-Segura, C. Zhou, B.E. Rittmann, P. Westerhoff "Comparing methods to deposit Pd-In catalysts on hydrogen-permeable hollow-fiber membranes for nitrate reduction" Water Research (2023) DOI: 10.1016/j.watres.2023.119877
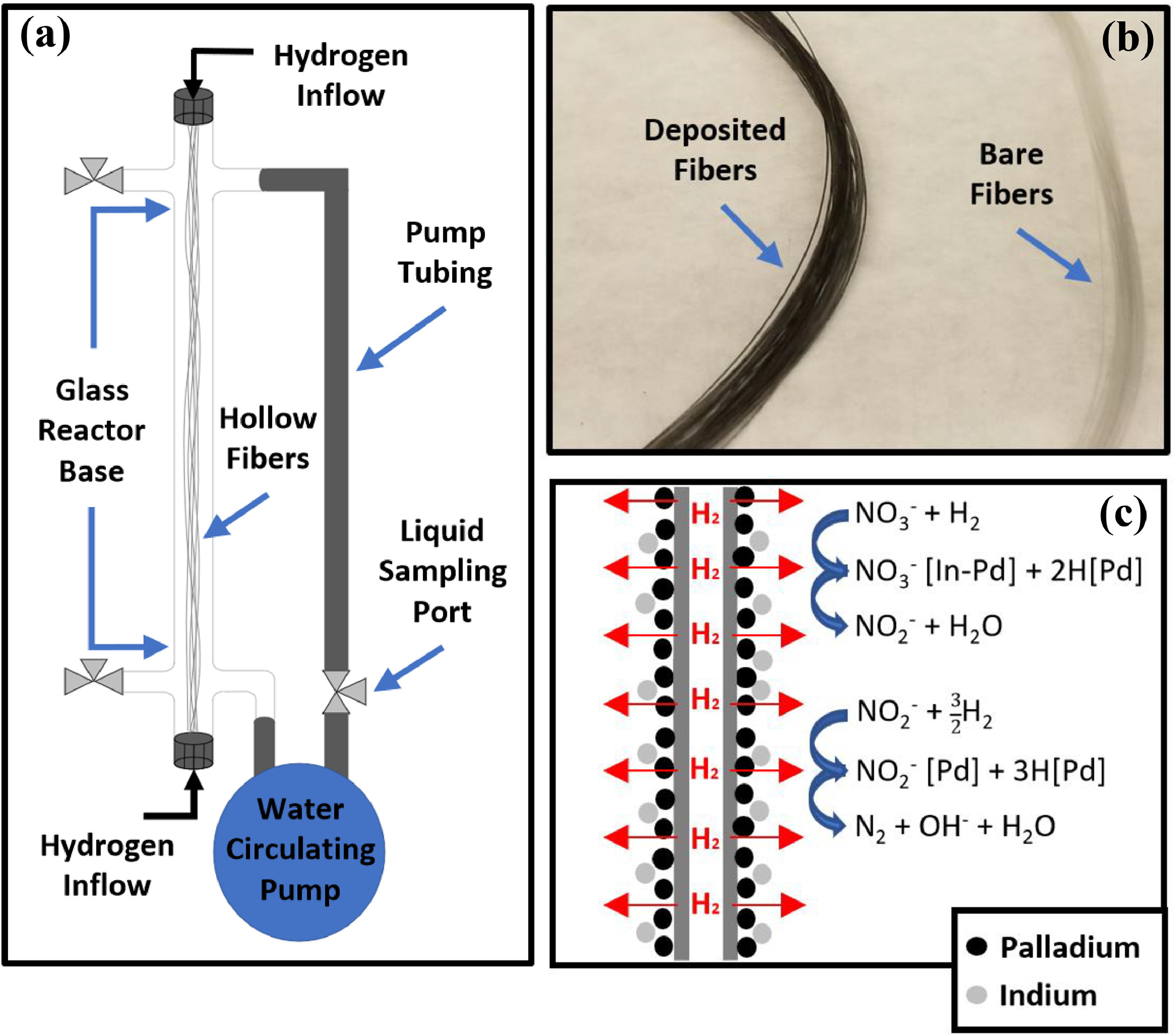
Catalytic hydrogenation of nitrate in water has been studied primarily using nanoparticle slurries with constant hydrogen-gas (H2) bubbling. Such slurry reactors are impractical in full-scale water treatment applications because 1) unattached catalysts are difficult to be recycled/reused and 2) gas bubbling is inefficient for delivering H2. Membrane Catalyst-film Reactors (MCfR) resolve these limitations by depositing nanocatalysts on the exterior of gas-permeable hollow-fiber membranes that deliver H2 directly to the catalyst-film. The goal of this study was to compare the technical feasibility and benefits of various methods for attaching bimetallic palladium/indium (Pd/In) nanocatalysts for nitrate reduction in water, and subsequently select the most effective method. Four Pd/In deposition methods were evaluated for effectiveness in achieving durable nanocatalyst immobilization on the membranes and repeatable nitrate-reduction activity: (1) In-Situ MCfR-H2, (2) In-Situ Flask-Synthesis, (3) Ex-Situ Aerosol Impaction-Driven Assembly, and (4) Ex-Situ Electrostatic. Although all four deposition methods achieved catalyst-films that reduced nitrate in solution (≥ 1.1 min−1gPd−1), three deposition methods resulted in significant palladium loss (>29%) and an accompanying decline in nitrate reactivity over time. In contrast, the In-Situ MCfR-H2 deposition method had negligible Pd loss and remained active for nitrate reduction over multiple operational cycles. Therefore, In-Situ MCfR-H2 emerged as the superior deposition method and can be utilized to optimize catalyst attachment, nitrate-reduction, and N2 selectivity in future studies with more complex water matrices, longer treatment cycles, and larger reactors.
179. H.P. Jacobs, W.C. Elias, K.N. Heck, D.P. Dean, J.J. Dodson, W. Zhang, J.H. Arredondo, C.J. Breckner, K. Hong, C.R. Botello, L. Chen, S.G. Mueller, S.R. Alexander, J.T. Miller, and M.S. Wong "Impregnation of KOAc on PdAu/SiO2 causes Pd-acetate formation and metal restructuring" Journal of Materials Chemistry A (2023) DOI: 10.1039/D3TA00820G
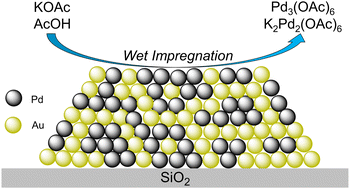
Potassium-promoted, oxide-supported PdAu is catalytically active for the gas-phase acetoxylation of ethylene to form vinyl acetate monomer (VAM), in which the potassium improves long-term activity and VAM selectivity. The alkali metal is incorporated into the catalyst via wet impregnation of its salt solution, and it is generally assumed that this common catalyst preparation step has no effect on the catalyst structure. However, in this work, we report evidence to the contrary. We synthesized a silica-supported PdAu (PdAu/SiO2, 8 wt% Pd, 4 wt% Au) model catalyst containing Pd-rich PdAu alloy and pure Au phases. Impregnation with potassium acetate (KOAc) aqueous solution and subsequent drying did not cause XRD-detectible changes to the bimetal structure. However, DRIFTS indicated the presence of Pd3(OAc)6 species, which is correlated to up to 2% Pd loss after washing of the dried KOAc-promoted PdAu/SiO2. Carrying out the impregnation step with an AcOH-only solution and subsequent drying caused significant enlargement of the pure Au grain size and generated a smaller amount of Pd3(OAc)6. During co-impregnation of AcOH and KOAc, grain sizes were enlarged slightly, and substantial amounts of K2Pd2(OAc)6 and Pd3(OAc)6 were detected by DRIFTS and correlated to up to 32% Pd loss after washing. Synchrotron XAS analysis showed that approximately half the Pd atoms were oxidized, corroborating the presence of the Pd-acetate species. These results indicate wet-impregnation-induced metal leaching can occur and be substantial during catalyst preparation.
178. S. Yin, J.F. López, J.J.C. Solís, M.S. Wong and D. Villagrán "Enhanced adsorption of PFOA with nano MgAl2O4@CNTs: influence of pH and dosage, and environmental conditions" Journal of Hazardous Materials Advances 9, 100252 (2023) DOI: 10.1016/j.hazadv.2023.100252
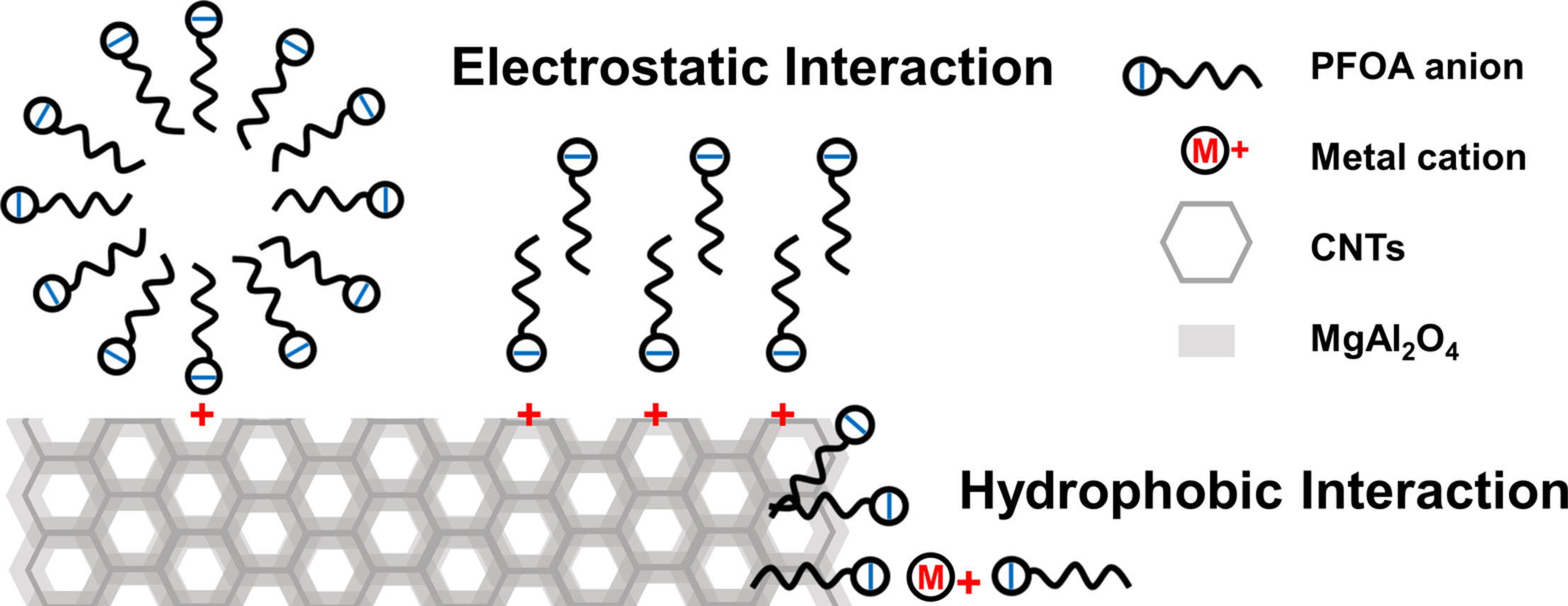
Perfluorooctanoic acid (PFOA) has received extensive attention due to its widespread distribution in the environment and concerns of its exposure to human health. Nano-MgAl2O4 modified carbon nanotubes (CNTs) were synthesized, characterized, and used as nanoadsorbents to remove ppb (μg/L)-levels of PFOA from drinking water and brackish groundwater. Nano-MgAl2O4@CNTs composite materials were characterized by UV-Vis, FT-IR, DLS, p-XRD, BET, and SEM with EDX. The adsorption isotherms and kinetic studies were fitted to a Freundlich and to a pseudo-second-order models, respectively. Composite nano-MgAl2O4@CNTs remove over 99% of PFOA (100 ppb) from water in 3 hours, and completely (100%) in 3.5 hours. The optimal pH range is under mild alkaline conditions (pH = 7.5-9.0). Electrostatic and hydrophobic interactions drive the PFOA adsorption onto MgAl2O4@CNTs. The adsorption data of ground and drinking water samples indicated that nano-MgAl2O4@CNTs is an efficient nanoadsorbent for PFOA removal.