2007 Abstracts.
41. M. A. Yaseen, J. Yu, M. S. Wong, and B. Anvari, "Laser-Induced Heating of Dextran-Coated Mesocapsules Containing Indocyanine Green" Biotechnol. Prog., 23 (6), 1431-1440 (2007). DOI:10.1021/bp0701618
Indocyanine green (ICG) is a photosensitive reagent with clinically relevant diagnostic and therapeutic applications. Recently, ICG has been investigated for its utility as an exogenous chromophore during laser-induced heating. However, ICGapos;s effectiveness remains hindered by its molecular instability, rapid circulation kinetics, and nonspecific systemic distribution. To overcome these limitations, we have encapsulated ICG within dextran-coated mesocapsules (MCs). Our objective in this study was to explore the ability of MCs to induce thermal damage in response to laser irradiation. To simulate tumorous tissue targeted with MCs, cylindrical phantoms were prepared consisting of gelatin, intralipid emulsion, and various concentrations of MCs. The phantoms were embedded within fresh chicken breast tissue representing surrounding normal tissue. The tissue models were irradiated at = 808 nm for 10 min at constant power (P = 4.2 W). Five hypodermic thermocouples were used to record the temperature at various depths below the tissue surface and transverse distances from the laser beam central axis during irradiation. Temperature profiles were processed to remove the baseline temperature and influence of light absorption by the thermocouple and subsequently used to calculate a damage index based on the Arrhenius damage integral. Tissue models containing MCs experienced a maximum temperature change of 18.5 °C. Damage index calculations showed that the heat generation from MCs at these parameters is sufficient to induce thermal damage, while no damage was predicted in the absence of MCs. ICG maintains its heat-generating capabilities in response to NIR laser irradiation when encapsulated within MCs. Such encapsulation provides a potentially useful methodology for laser-induced therapeutic strategies.
40. R. S. Krishnan, M. E. Mackay, P. M. Duxbury, C. J. Hawker, S. Asokan, M. S. Wong, R. Goyette, and P. Thiyagarajan, "Improved Polymer Thin-film Wetting Behavior through Nanoparticle Segregation to Interfaces" J. Phys. Condens. Matter, 19, Art. No. 356003 (2007) DOI:10.1088/0953-8984/19/35/356003
We report a systematic study of improved wetting behavior for thin polymer films containing nanoparticles, as a function of nanoparticle size and concentration, the energy of the substrate and the dielectric properties of the nanoparticles. An enthalpy matched system consisting of polystyrene nanoparticles in linear polystyrene is used to show that nanoparticles are uniformly distributed in the film after spin coating and drying. However, on annealing the film above its bulk glass transition temperature these nanoparticles segregate strongly to the solid substrate. We find that for a wide range of film thicknesses and nanoparticle sizes, a substrate coverage of nanoparticles of approximately a monolayer is required for dewetting inhibition. Cadmium selenide quantum dots also inhibit dewetting of polystyrene thin films, again when a monolayer is present. Moreover, TEM microscopy images indicate that CdSe quantum dots segregate primarily to the air interface. Theoretical interpretation of these phenomena suggests that gain of linear chain configurational entropy promotes segregation of nanoparticles to the solid substrate, as occurs for polystyrene nanoparticles; however, for CdSe nanoparticles this is offset by surface energy or enthalpic terms which promote segregation of the nanoparticles to the air interface.
39. W. C. Ketchie, Y. L. Fang, M. S. Wong, M. Murayama and R. J. Davis, "Influence of gold particle size on the aqueous-phase oxidation of carbon monoxide and glycerol" J. Catal. 250, 94-101 (2007). DOI:10.1016/j.jcat.2007.06.001
Carbon-supported Au particles with mean sizes ranging from 5 to 42 nm and unsupported Au powder were evaluated as catalysts in the aqueous-phase oxidation of CO and glycerol. For the aqueous-phase oxidation of CO at pH 14 and 300 K, the turnover frequency (TOF) for the 5-nm Au particles was 5 s-1, whereas the TOF for large supported Au (42 nm) and bulk Au were only 0.5 and 0.4 s-1, respectively. The observed rate of
peroxide formation during CO oxidation also was much higher on the small Au particles. Oxidation of glycerol in the aqueous phase at 333 K and elevated pH over the same catalysts revealed a similar influence of particle size, with the 5-nm Au particles giving a TOF of 17 s-1 at pH 13.8 and the larger particles and bulk Au nearly an order of magnitude less active. However, large Au particles (>20 nm) were more selective to glyceric
acid. The lower selectivity of small Au particles is attributed to a higher formation rate of H2O2 during glycerol oxidation, because peroxide promotes CC cleavage reaction.
38. J. Sunarso, C-Y. Chen, A. T. T. Tran, M. S. Wong and J. C. Diniz Da Costa, "Proton conductive composite membranes" Int. J. Nanotech. 4, 597-608 (2007). DOI:10.1504/IJNT.2007.014754
In this work we investigated the synthesis of composite organic and inorganic membranes for proton conduction. Particles derived from metal alkoxides (M(OR)n) sol-gel processes (Ti, Zr, W with phosphoric acid) were embedded in polymeric matrices of poly-vinyl alcohol, (3-glycidoxypropyl)-trimethoxysilane and ethylene glycol. The structure of the composite membranes was complex as several IR peaks were convoluted, indicating the assignment of several functional groups. However, the peaks assigned to OH groups reduced in intensity in the composite membranes, indicating that cross-linking of hydroxyl groups in the organic and inorganic phases of the membrane may have occurred. The particles allowed for re-arrangement of the polymer matrix, as crystallinity was reduced compared to a polymer blank membrane. The composite membrane process resulted in homogeneous dispersion of nanoparticles into the polymer film. Proton conduction of the inorganic phase was mainly dominated by titania. Binary mixtures of titania phosphate (sample name TiP) resulted in proton conduction of 7.15 × 10?2 S.cm?1, one order of magnitude higher than zirconia phosphate (ZrP). The addition of Zr and W to TiP forming ternary or quaternary phases also led to lower proton conduction as compared to TiP. Similar trends were also observed for the composite membranes, though the TiP composite membrane proton conduction reduced after several hours of testing at 50°C, which was mainly attributed to acid leaching.
37. S. Asokan, K. M. Krueger, V. L. Colvin, and M. S. Wong, "Shape-Controlled Synthesis of CdSe Tetrapods Using Cationic Surfactant Ligands" Small 3(7), 1164-1169 (2007). DOI: 10.1002/smll.200700120
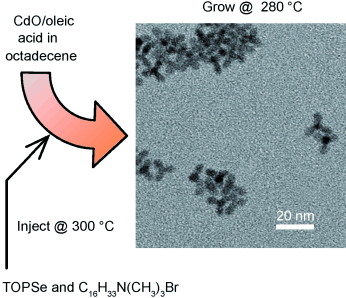
Keeping in shape: Quaternary alkylammonium compounds promote the formation of faceted quantum dots. Their use in hot-injection synthesis chemistry provides a new means to form uniform CdSe tetrapods without a selective precipitation step (see picture; TOPSe: trioctylphosphine selenide), and introduces the prospect of nanoparticle shape control through ligand-nanoparticle charge interactions.
36. A. Tuteja, M. E. Mackay, S. Narayanan, S. Asokan, and M. S. Wong, "Breakdown of the Continuum Stokes-Einstein Relation for Nanoparticle Diffusion" Nano Lett. 7, 1276-1281 (2007). DOI:10.1021/nl070192x
Cadmium selenide nanoparticles are found to diffuse approximately 200 times faster in a polymeric liquid than predicted by the Stokes?Einstein relation. This remarkable behavior is hypothesized to be due to the nanoparticles being smaller than the entanglement mesh to create a frictional drag that does not follow continuum expectations, in line with a theoretical calculation presented before. This is one of the first demonstrations of X-ray photo correlation spectroscopy applied to polymeric liquids, which we use to explain the simultaneous 60% viscosity reduction of the mixture through a proposed constraint release mechanism.
35. W. V. Knowles, M. O. Nutt, and M. S. Wong, "Supported Metal Oxides and the Surface Density Metric" in Handbook of Catalyst Synthesis: The Science and Engineering of Catalyst Preparation; J. R. Regalbuto, Ed.; Taylor and Francis: Boca Raton; Chapter 11, 251-281 (2007).
Supported metal oxides (SMOs) comprise a large class of catalytic materials used in numerous industrial processes. There are many conventional approaches to preparing these materials, ranging from impregnation and equilibrium adsorption to grafting and co-precipitation. Independent of preparation methods, one of the key metrics in characterizing SMOs is surface density, which quantifies the amount of the supported metal oxide relative to the underlying support surface area. Catalytic activity is correlated to the surface density-dependent structure of the supported species. There are different definitions for surface density and different methods for its determination, though, causing some difficulties in reconciling structure-activity results reported by different researchers. Here, a rigorous analysis of the different surface density calculation methods is presented, using tungstated zirconia as an example.
34. J. Yu, M. A. Yaseen, B. Anvari, and M. S. Wong, "Synthesis of Near-Infrared-Absorbing Nanoparticle-Assembled Capsules" Chem. Mater. 19, 1277-1284 (2007). DOI: 10.1021/cm062080x
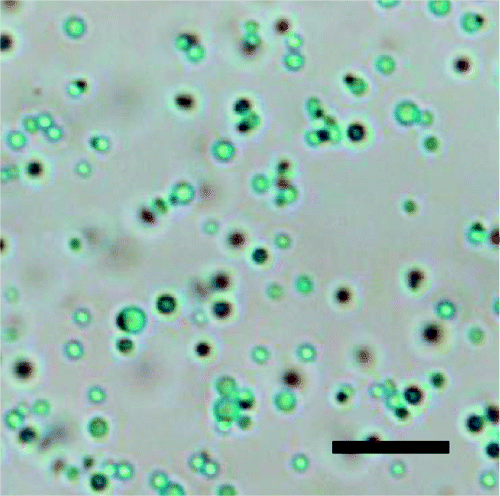
Indocyanine green (ICG) is an FDA-approved photosensitizer dye used in clinical settings for optical diagnostics and near-infrared laser-based therapy. However, the rapid clearance and nonspecific vascular plasma binding issues impede ICG performance. Encapsulating ICG within a colloidal matrix is a potential
approach to solving these problems, but thus far, there has been limited success. A new strategy, based on the nanoparticle assembly synthesis of stable, non-liposomal nanoparticle/polymer microcapsules, to encapsulate ICG is presented. Nanoparticle-assembled capsules (NACs) are prepared at room temperature, in aqueous solution,
and at neutral pH by combining a polyallylamine solution, a phosphate solution, and an aqueous sol of silica nanoparticles; ICG-containing NACs with 0.6?1.0 ?m diameter are prepared by adding an ICG solution before the nanoparticle sol. ICG loading is readily controlled with an attainable maximum loading
of  23 wt %. There is negligible leakage from the capsules after 24 h at room temperature in phosphate buffer saline solution, with 17% ICG leakage after 8 h at 37 °C. ICG-containing NACs are capable of heat generation in response to near-infrared
laser irradiation and are stable to multiple photothermal heating cycles. Fibroblast cells exposed to these capsules remain viable after 2 days of incubation. ICG-containing NACs are a promising material for new photothermal therapy applications and are illustrative of a new approach for encapsulating organic dye compounds.
23 wt %. There is negligible leakage from the capsules after 24 h at room temperature in phosphate buffer saline solution, with 17% ICG leakage after 8 h at 37 °C. ICG-containing NACs are capable of heat generation in response to near-infrared
laser irradiation and are stable to multiple photothermal heating cycles. Fibroblast cells exposed to these capsules remain viable after 2 days of incubation. ICG-containing NACs are a promising material for new photothermal therapy applications and are illustrative of a new approach for encapsulating organic dye compounds.
33. R. S. Krishnan, M. E. Mackay, P. M. Duxbury, A. Pastor, C. J. Hawker, B. Van Horn, S. Asokan, and M. S. Wong, "Self-Assembled Multilayers of Nanocomponents" Nano Lett. 7, 484-489 (2007). DOI:10.1021/nl062866u
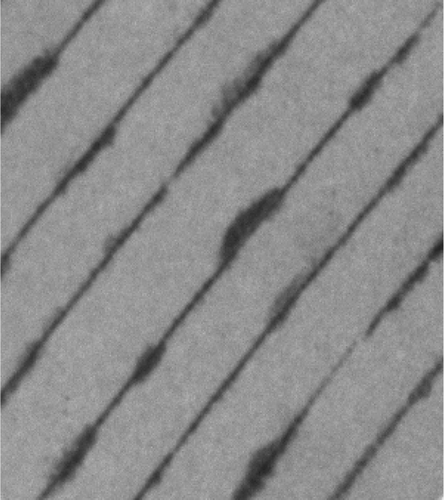
We show it is possible to assemble nanoparticle?polymer layers in a controllable manner dictated by the difference in nano-object morphology and dielectric properties. A thin (10?100 nm) layer of the two components is spin coated onto a solid substrate and the system thermally aged to activate a cross-linking process between polymer molecules. The nanoparticles segregate to the solid substrate prior to complete cross-linking if entropic forces are dominant or to the air interface if dielectric (surface energy) forces are properly tuned. Subsequent layers are then spin coated onto the layer below, and the process is repeated to create layered structures with nanometer accuracy useful for tandem solar cells, sensors, optical coatings, etc. Unlike other self-assembly techniques the layer thicknesses are dictated by the spin coating conditions and relative concentration of the two components.
32. P. R. LeDuc, M. S. Wong, et al., "Towards an in vivo Biologically Inspired Nanofactory" Nature Nanotech., 2, 3 - 7 (2007). DOI: 10.1038/nnano.2006.180
Nanotechnology is having a major impact on medicine and the treatment of disease, notably in imaging and targeted drug delivery. It may, however, be possible to go even further and design 'pseudo-cell' nanofactories that work with molecules already in the body to fight disease.