|
Reviews in Undergraduate Research - Issue 2
SUMMARY Macrophages play an integral role in
the immune system. They are professional phagocytes, responsible for
the recognition and engulfment of pathogens and toxins. Usually, phagocytosis
of pathogens leads to macrophage activation, inducing the release
of pro-inflammatory cytokines such as IL1, IL6 and TNF, as well as
other toxic mediators that cause non-specific tissue damage. In addition
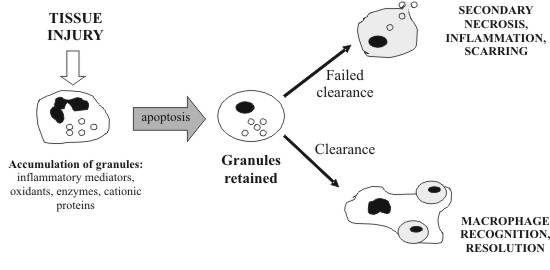
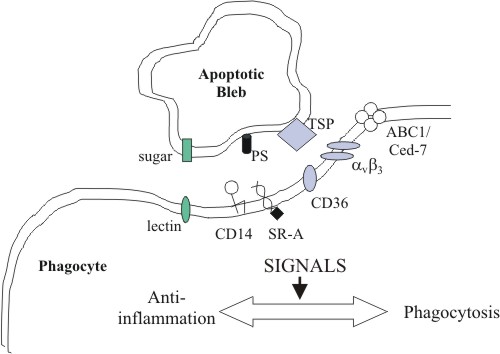
to their immune functions, macrophages also participate MACROPHAGES AND APOPTOTIC CLEARANCE Macrophages express a variety of receptors for pathogen associated molecular pathways (PAMPs) such as lipopolysaccharide (LPS) and mannose receptors. These enable the recognition and phagocytosis of a large repertoire of pathogens, and the subsequent generation of inflammation to eradicate the infection. Interestingly, many of the same receptors are also involved in the recognition and clearing of self-apoptotic cells. Yet, instead of macrophage activation and production of pro-inflammatory cytokines, anti-inflammatory mediators are released, and clearing is quickly and painlessly performed. However, mouse peritoneal macrophages ingesting apoptotic T cells have been observed to secrete the proinflammatory chemokine MIP-2, suggesting that some degree of macrophage activation may occur under certain circumstances (Uchimura et al., 1997). If the rate of apoptosis exceeds the clearing capacity of macrophages, apoptotic cells may become necrotic, resulting in the release of harmful cellular contents and damage to surrounding tissue (fig 1). Treatment of mice with anti-Fas antibody triggered a massive wave of apoptosis in the liver, leading to extensive hepatic necrosis and death (Ogasawara et al., 1993). Hence, clearance is a crucial step in the resolution of apoptosis and containment of cell death. The exact interactions between apoptotic cells and macrophages have not been fully elucidated, but it appears to involve multiple, partially redundant pathways. Apart from the well-known phosphatidylserine (PS) 'eat-me' signal (Fadok et al., 1992; Fadok et al., 2001; Schlegel and Williamson, 2001), other surface markers of apoptotic cells have not been well characterised. On the other hand, macrophage receptors are better known, and several promising candidates have been identified, including scavenger receptors, CD36, CD14 and the PS receptor (fig 2).
CLASS A SCAVENGER RECEPTORS The scavenger receptors (SRs) are a structurally diverse family of receptors with broad ligand specificities. SR-A binds acetylated and oxidised low-density lipoprotein (LDL), and polyanionic compounds such as maleylated bovine serum albumin and polyinosinic acid (Pearson, 1996). The addition of monoclonal antibodies to SR-A, or polyanionic ligands significantly inhibits the in vitro phagocytosis of apoptotic thymocytes by thymus-derived macrophages (Platt, 1996). Further studies with thymic macrophages from SR-A null mice show that although phagocytosis of thymocytes was inhibited by 50%, the relative number of apoptotic thymocytes in these mice was not appreciably larger. This suggests that other receptors are sufficient for normal apoptotic clearance in the thymus (Platt, 1998). CLASS B SCAVENGER RECEPTORS AND THE VITRONECTIN RECEPTOR The class B scavenger receptor CD36 recognises a range of ligands, including typeI collagen, thrombospondin, oxidised LDL and PS (Rigotti, 1995). It is observed that the plasma protein thrombospondin 1 (TSP1) acts to bridge apoptotic cells, CD36 (a TSP1 receptor) and the vitronectin receptor (αvß3 integrin) (Savill et al., 1990; Savill et al., 1992; Stern et al., 1996; Akbar, 1994). CD36 gene transfer to amateur phagocytes such as melanoma cells boosts their capacity for uptake of apoptotic neutrophils to near-professional levels. Antibodies to αvß3 and TSP1 inhibit this improvement (Ren et al., 1995). Conflicting evidence exists for the importance of CD36 in phagocytizing apoptotic cells in vivo. On one hand, blood monocytes from SLE patients demonstrate a decrease in CD36 levels paralleled by a deficiency in the phagocytosis of apoptotic cells. On the other hand, monocyte-derived macrophages from CD36-deficient patients show no defect in the phagocytosis of apoptotic neutrophils (Platt, 1998). This reflects the considerable redundancy that underlies the uptake of apoptotic cells.
CD14 A monoclonal antibody that
specifically inhibited internalisation of intact ageing neutrophils
(PMNs) by monocyte-derived macrophages was found to recognise CD14,
a molecule also known to recognise LPS. Although apoptotic cells bind
to CD14 at a site close to the LPS-binding site, they do not induce
the severe inflammation produced by LPS (Devitt, 1998). LPS normally
binds CD14 in the presence of serum LPS binding protein (LBP). The
complex then interacts with macrophage toll-like receptor 2 (TLR2)
to result in macrophage activation (Yang, 1998). It is possible that
CD14 does not interact with TLR2 during apoptotic clearing due to
the absence of LBP, thus triggering a different response. PS RECEPTORS It is widely accepted that phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane is an important signal displayed by preapoptotic cells (Fadok et al., 1992; Fadok et al., 2001; Schlegel and Williamson, 2001). Macrophage phagocytosis of apoptotic lymphocytes was inhibited, in a dose-dependent manner, by liposomes containing phosphatidyl- L-serine, but not by liposomes containing other anionic phospholipids, including phosphatidyl- D-serine (Fadok et al., 1992). Yet, the nature and identity of the macrophage PS receptor had remained a mystery. A recent publication by Fadok et al reports the cloning of a candidate receptor, identified using monoclonal antibodies against human macrophages. Transfection of the gene into Jurkat T cells (which are negative for the receptor) confers the ability to bind and engulf apoptotic cells. PS liposomes, but not phophatidylinositol (PI) nor phosphatidylcholine (PC) liposomes, inhibit the binding, suggesting that the cloned protein is a PS-specific receptor involved in the recognition and engulfment of apoptotic cells (Fadok et al., 2000). Furthermore, stimulation of this receptor on different types of phagocytes by apoptotic cells, PS- containing liposomes or an IgM monoclonal anti-PS antibody initiates release of TGF-ß, known to be involved in the anti-inflammatory effects of apoptotic cells (Fadok et al., 2001). Another protein implicated in PS recognition is milk fat globule-EGF-factor 8 (MFG-E8) (Hanayama, 2002). A purified recombinant form, MFG-E8-L, binds to thymocytes treated with dexamethasone, which induces apoptosis. This interaction is reduced by pre-treatment of the cells by Annexin V, which binds PS. MFG-E8-L was found to bind to PS and PE, but not PC and PI. It also contains an arginine-glycine-aspartate (RGD) motif, which can be recognised by integrins. MFG-E8-L enhances phagocytosis of apoptotic thymocytes by mouse NIH3T3 cells, especially those NIH3T3 cells transformed to express a high level of αvß3 integrin. Integrins have been suggested as receptors for apoptotic cells in several systems (Savill et al., 1990; Albert et al., 1998). However, because neither αvß3 or αvß5 integrin can bind PS, it has not been clear how these integrins recognize apoptotic cells. MFG-E8-L seems to resolve this dilemma. Multiple receptors appear to respond to PS exposure. Pradhan et al demonstrated that both activated and unactivated macrophages recognize PS, but with different receptor systems. Phagocytosis of apoptotic lymphocytes by activated (but not by unactivated) macrophages is inhibited by pure PS vesicles as well as by N- acetylglucosamine, implicating involvement of a lectin-like receptor in this case. Conversely, uptake of apoptotic lymphocytes by unactivated (but not by activated) macrophages is inhibited by PS on the surface of erythrocytes as well as by the tetrapeptide RGDS and cationic amino acids and sugars, implicating involvement of the vitronectin receptor in this case. Recognition by both classes of macrophages is blocked by the monocyte-specific monoclonal antibody 61D3. The signal recognized by activated macrophages appears to develop on the lymphocyte prior to assembly of the signal recognized by unactivated macrophages. Collectively, these results suggest that PS exposure on the surface of apoptotic lymphocytes generates a complex and evolving signal recognized by different receptor complexes on activated and unactivated macrophages (Pradhan, 1997). Fadok et al proposes that
the myriad of macrophage receptors may provide the strong adhesion
needed to increase the likelihood of contact between the PS Receptor
and its phospholipid ligand, which is required for uptake (Fadok et
al., 2001). Interestingly, PS is exposed on both apoptotic and necrotic
cells, and is not responsible for the differential macrophage responses
invoked upon phagocytosis. Necrotic cells, when recognized, enhance
proinflammatory responses of activated macrophages, although they
are not sufficient to trigger macrophage activation. In marked contrast,
apoptotic cells profoundly inhibit phlogistic macrophage responses;
this represents a cell-associated, dominant-acting anti-inflammatory
signaling activity acquired posttranslationally during the process
of physiological cell death (Cocco and Ucker, 2001). GENETIC PARALLELS IN C. elegans Although apoptotic clearance
in C. elegans is performed by neighbouring, non-professional cells,
genetic analyses has revealed two partially redundant engulfment pathways
which might be applicable to macrophages. The two groups are ced-1,
ced-6 and ced-7, and ced-2, ced-5, ced-10 and ced-12 (Ellis et al.,
1991). ced-1, ced-6 and ced-7 encode a scavenger receptor-like protein,
a PTB-domain containing adaptor protein and an ABC transporter, respectively.
They are components of a conserved signalling pathway in phagocytic
cells, required for the recognition of an unknown cell-corpse signal
(Hengartner, 2001). ced-7 also functions in apoptotic cells, where
it might be involved in the generation of this signal. ced-2, ced-5,
ced-10 and ced-12 encode homologues of mammalian CrkII, Dock180, Rac
and ELMO, which function to transduce another unknown cell-corpse
signal to the actin cytoskeleton of the phagocytic cell (Conradt,
2001). PHAGOCYTOSIS AND THE INITIATION OF APOPTOSIS In most cases, blocking
engulfment or eliminating phagocytes does not prevent the demise of
doomed cells. However, some exceptions have been observed. For example,
activated macrophages cocultured with myofibroblast-like mesangial
cells in vitro can cause these cells to die (Duffield et al., 2000).
In addition, macrophage elimination prevents capillary regression
in the rat's eye in vivo, but reconstitution with unactivated macrophages
restores the normal phenotype (Lang and Bishop, 1993; Diez-Roux and
Lang, 1997). Finally, the death of the male-specific linker cell in
C. elegans is prevented by inactivating one of the two engulfment
pathways or by ablating the two neighbouring cells normally responsible
for the engulfment of this cell. Thus, it appears that phagocytes
are capable of inducing apoptosis. In mammalian systems, mediators
of macrophage cytotoxicity in vitro include TNF- van de Loosdrecht,
1993) and nitric oxide (Cui, 1994). POSITIVE FEEDBACK CYCLE BETWEEN PHAGOCYTES AND APOPTOTIC CELLS Recent work in C. elegans has led to the discovery of a positive feedback loop between the engulfment machinery in phagocytic cells and the cell-death machinery in apoptotic cells. It was demonstrated that mutations that block engulfment strongly enhance the ability of partial lf (loss of function) mutations of pro-apoptotic genes (ced-3 [caspase], ced-4 [CED-3 activator], egl-1 [CED-4 activator]), hence rescuing cells destined to die by apoptosis (Reddien et al., 2001; Hoeppner, 2001). This is reversed upon expression of the corresponding wild-type engulfment gene in the phagocytic cell (Reddien et al., 2001). But engulfment mutations in otherwise wild type, or ced-3 null worms do not enhance cell survival. It suggests that the effect is dependent on the induction of at least low levels of CED-3, and plays an important role only when the cell-death machinery is compromised (Reddien et al., 2001). Hence, it can be surmised that CED-3 is initially induced to a level sufficient to induce morphological changes and the exposure of eat-me signals, but not sufficient to ensure the completion of apoptosis. The engulfment machinery then positively feeds back to guarantee death of the cell (fig 1). This means that cells in which only low levels of caspases have been activated (and undergone morphological changes) can still be rescued to form fully differentiated, functional cells (Reddien et al., 2001; Hoeppner, 2001). Blocks in apoptosis and
engulfment are non-lethal in laboratory-grown C. elegans, but can
be detrimental in higher organisms (Ren and Savill, 1998) as well,
to ensure proper functioning and control of apoptosis. More work has
to be done to elucidate the details of the feedback mechanism in C.
elegans, and to identify homologous pathways in higher organisms. DYNAMIC RELATIONSHIP BETWEEN PHAGOCYTES AND CELLS COMMITTED TO DIE Other experiments have
shown that not only do phagocytes determine the fate of apoptotic
cells, dying cells can also affect the survival of phagocytes (Tepass
et al., 1994). In Drosophila, apoptotic clearance is performed by
professional phagocytes, which are differentiated from haemocytes.
Mutants that lack haemocytes can still initiate and complete apoptosis.
But apoptosis-incompetent mutants (due to elimination of important
pro-apoptotic genes hid, reaper and grim) fail to produce phagocytes
from haemocytes, and do not express the CD36-like scavenger receptor
Croquemort. Conversely, the induction of ectopic apoptosis results
in an increased number of phagocytes (Zhou et al., 1995; Franc et
al., 1999). Therefore, the differentiation of haemocytes into phagocytes
in Drosophila is dependent on the presence of apoptotic cells. CONCLUSIONS Macrophage clearance of
apoptotic bodies is crucial in higher organisms. It is efficient and
non-inflammatory, hence limiting and controlling cell death. Although
the exact details of apoptotic eat-me signals and the engulfment machinery
have not been elucidated, PS, the PS receptor, scavenger receptors,
CD14 and integrins play central roles. Drosophila and C. elegans provide
useful models for work in this field. Recent evidence suggests the
presence of a positive feedback loop between the cell-death and engulfment
machineries. It is increasingly evident that complex interactions
exist between phagocytes and apoptotic cells. Not only might phagocytes
initiate and regulate apoptosis, apoptotic cells may also influence
the fate of phagocytes. More work is required before we can better
understand the nature of this relationship, and engineer possible
therapeutic applications. ABOUT THE AUTHOR The author wrote this paper as a final term project while studying abroad at St. Catherine's College, Oxford University. She will graduate this May from Johns Hopkins University, and will go on to Stanford University for her PhD in Immunology. FURTHER READING Fadok VA (1999) Clearance: the last and often forgotten stage of apoptosis. J Mammary Gland Biol Neopl 4:203-211 (Review Article). Reddien PW, Cameron S, Horvitz HR. (2001) Phagocytosis promotes programmed cell death in C. elegans. Nature 412:198-202 (New Theories about macrophage initiation of apoptosis). REFERENCES
Albert ML, Sauter B, Bhardwaj N. (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392, 86-89. Cocco RE, Ucker DS. (2001) Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 12, 919-930. Conradt B. (2001) Cell engulfment, no sooner ced than done. Dev Cell. 1, 445-447. Cui S, Reichner JS, Mateo RB, Albina JE. (1994) Activated murine macrophages induce apoptosis in tumor cells through nitric oxide dependent or -independent mechanisms. Cancer Research. 54, 2462-2467. Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 392, 505-509. Diez-Roux G, Lang RA. (1997) Macrophages induce apoptosis in normal cells in vivo. Development. 124, 3633-3638. Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS. (2000) Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 164, 2110-2119. Ellis RE, Jacobson DM, Horvitz HR. (1991) Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 129, 79-94. Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 148, 2207-2216. Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 405, 85-90. Fadok VA, Xue D, Henson P. (2001) If phosphatidylserine is the death knell, a new phosphatidylserine-specific receptor is the bellringer. Cell Death Differ. 8, 582-587. Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol Chem. 276, 1071-1077. Franc NC, Heitzler P, Ezekowitz RA, White K. (1999) Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 284, 1991-1994. Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. (2002) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 417, 182-187. Hengartner MO. (2001) Apoptosis: corralling the corpses. Cell. 104, 325-328. Hoeppner DJ, Hengartner MO, Schnabel R. (2001) Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 412, 202-206. Lang RA, Bishop JM. (1993) Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 74, 453-462. Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. (1993) Lethal effect of the anti- Fas antibody in mice. Nature (London) 364, 806-809. Pearson AM. (1996) Scavenger receptors in innate immunity. Curr. Opin. Immunol. 8, 20-28. Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. (1996) Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA 93, 12456-12460. Platt N, da-Silva RP, Gordon S. (1998) Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 8, 365- 372. Pradhan D, Krahling S, Williamson P, Schlegel RA. (1997) Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 8, 767-778. Reddien PW, Cameron S, Horvitz HR. (2001) Phagocytosis promotes programmed cell death in C. elegans. Nature. 412, 198-202. Ren Y, Silverstein RL, Allen J, Savill J. (1995) CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 181, 1857-1862. Ren Y, Savill J. (1998) Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563-568. Rigotti A, Acton SL, Krieger M. (1995) The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 270, 16221-16224. Savill J, Dransfield I, Hogg N, Haslett C. (1990) Vitronectin receptor mediated phagocytosis of cell undergoing apoptosis. Nature. 343, 170-173. Savill J, Hogg N, Ren Y, Haslett C. (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513-1522. Schlegel RA, Williamson
P. (2001) Phosphatidylserine, a death knell. Cell Death Differ. 8,
551-563. Stern M, Savill J, Haslett C. (1996) Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis: Mediation by avß3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am. J. Pathol. 149, 911-921. Tepass U, Fessler LI, Aziz A, Hartenstein V. (1994) Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 120, 1829-1837. Uchimura E, Kodaira T, Kurosaka K, Yang D, Watanabe N, Kobayashi Y. (1997) Interaction of phagocytes with apoptotic cells leads to production of pro-inflammatory cytokines. Biochem. Biophys. Res. Commun. 239, 799-803. van de Loosdrecht AA, Ossenkoppele GJ, Beelen RHJ, Broekhoven MG, Drager AM, Langenhuijsen MMAC. (1993) Apoptosis in tumor necrosis factor-a-dependent, monocyte-mediated leukemic cell death: a functional, morphologic and flow-cytometric analysis. Exp. Hematol. 21, 1628-1639. Vivers S, Dransfield I, Hart SP. (2002) Role of macrophage CD44 in the disposal of inflammatory cell corpses. Clin Sci (Lond) 103, 441-449. Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. (1998) Toll-like receptor-2 mediates lipopolysacharide-induced cellular signalling. Nature. 395, 284-288. Zhou L, Hashimi H, Schwartz LM, Nambu JR. (1995) Programmed cell death in the Drosophila central nervous system midline. Curr. Biol. 5, 784-790. |
|
RUR
- Issue 2 |